Page 190 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 190
6-1 ELECTROCHEMICAL BIOSENSORS 175
liberates the enzyme. At a ®xed enzyme concentration, the rate v of the enzyme-
catalyzed reaction is given by the Michaelis±Menten equation:
V S
m
v
6-3
K S
m
where K m is the Michaelis±Menten constant and V m is the maximum rate of the
reaction. The term K corresponds to the substrate concentration for which the rate
m
is equal to half of V . In the construction of enzyme electrodes, it is desirable to
m
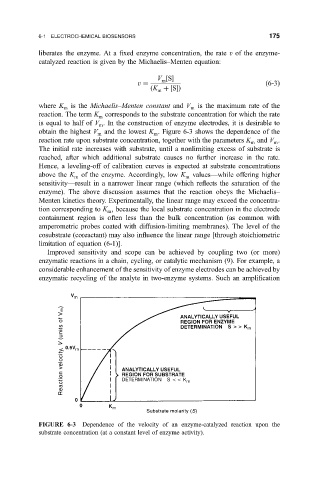
obtain the highest V and the lowest K . Figure 6-3 shows the dependence of the
m
m
reaction rate upon substrate concentration, together with the parameters K and V .
m
m
The initial rate increases with substrate, until a nonlimiting excess of substrate is
reached, after which additional substrate causes no further increase in the rate.
Hence, a leveling-off of calibration curves is expected at substrate concentrations
above the K of the enzyme. Accordingly, low K valuesÐwhile offering higher
m m
sensitivityÐresult in a narrower linear range (which re¯ects the saturation of the
enzyme). The above discussion assumes that the reaction obeys the Michaelis±
Menten kinetics theory. Experimentally, the linear range may exceed the concentra-
tion corresponding to K , because the local substrate concentration in the electrode
m
containment region is often less than the bulk concentration (as common with
amperometric probes coated with diffusion-limiting membranes). The level of the
cosubstrate (coreactant) may also in¯uence the linear range [through stoichiometric
limitation of equation (6-1)].
Improved sensitivity and scope can be achieved by coupling two (or more)
enzymatic reactions in a chain, cycling, or catalytic mechanism (9). For example, a
considerable enhancement of the sensitivity of enzyme electrodes can be achieved by
enzymatic recycling of the analyte in two-enzyme systems. Such an ampli®cation
FIGURE 6-3 Dependence of the velocity of an enzyme-catalyzed reaction upon the
substrate concentration (at a constant level of enzyme activity).