Page 191 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 191
176 ELECTROCHEMICAL SENSORS
scheme generates more than a stoichiometric amount of product and hence large
analytical signals for low levels of the analyte. In addition, a second enzyme can be
used to generate a detectable (electroactive) species, from a nonelectroactive product
of the ®rst reaction.
6-1.1.2 Enzyme Electrodes of Analytical Signi®cance
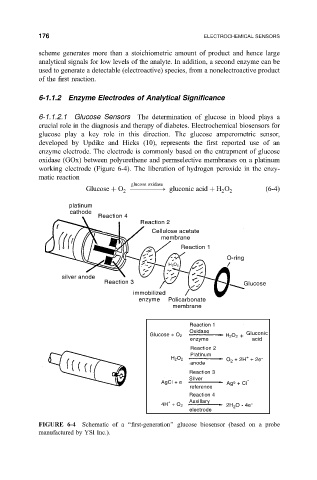
6-1.1.2.1 Glucose Sensors The determination of glucose in blood plays a
crucial role in the diagnosis and therapy of diabetes. Electrochemical biosensors for
glucose play a key role in this direction. The glucose amperometric sensor,
developed by Updike and Hicks (10), represents the ®rst reported use of an
enzyme electrode. The electrode is commonly based on the entrapment of glucose
oxidase (GOx) between polyurethene and permselective membranes on a platinum
working electrode (Figure 6-4). The liberation of hydrogen peroxide in the enzy-
matic reaction
glucose oxidase
Glucose O 2 ! gluconic acid H O 2
6-4
2
FIGURE 6-4 Schematic of a ``®rst-generation'' glucose biosensor (based on a probe
manufactured by YSI Inc.).