Page 197 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 197
182 ELECTROCHEMICAL SENSORS
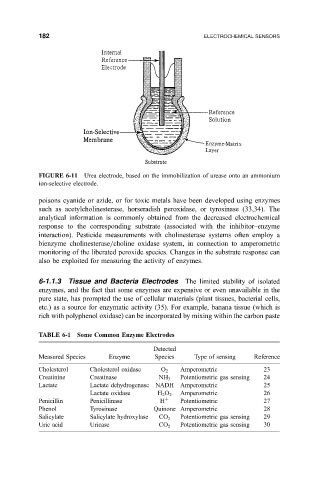
FIGURE 6-11 Urea electrode, based on the immobilization of urease onto an ammonium
ion-selective electrode.
poisons cyanide or azide, or for toxic metals have been developed using enzymes
such as acetylcholinesterase, horseradish peroxidase, or tyrosinase (33,34). The
analytical information is commonly obtained from the decreased electrochemical
response to the corresponding substrate (associated with the inhibitor±enzyme
interaction). Pesticide measurements with cholinesterase systems often employ a
bienzyme cholinesterase=choline oxidase system, in connection to amperometric
monitoring of the liberated peroxide species. Changes in the substrate response can
also be exploited for measuring the activity of enzymes.
6-1.1.3 Tissue and Bacteria Electrodes The limited stability of isolated
enzymes, and the fact that some enzymes are expensive or even unavailable in the
pure state, has prompted the use of cellular materials (plant tissues, bacterial cells,
etc.) as a source for enzymatic activity (35). For example, banana tissue (which is
rich with polyphenol oxidase) can be incorporated by mixing within the carbon paste
TABLE 6-1 Some Common Enzyme Electrodes
Detected
Measured Species Enzyme Species Type of sensing Reference
Cholesterol Cholesterol oxidase O 2 Amperometric 23
Creatinine Creatinase NH 3 Potentiometric gas sensing 24
Lactate Lactate dehydrogenase NADH Amperometric 25
Lactate oxidase H 2 O 2 Amperometric 26
Penicillin Penicillinase H Potentiometric 27
Phenol Tyrosinase Quinone Amperometric 28
Salicylate Salicylate hydroxylase CO 2 Potentiometric gas sensing 29
Uric acid Uricase CO 2 Potentiometric gas sensing 30