Page 201 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 201
186 ELECTROCHEMICAL SENSORS
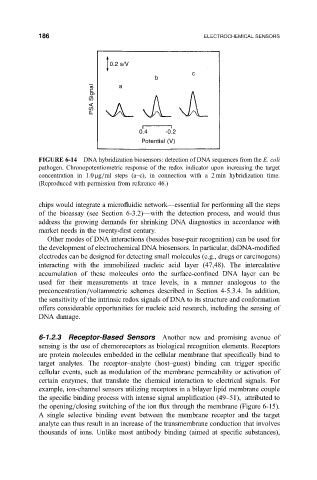
FIGURE 6-14 DNA hybridization biosensors: detection of DNA sequences from the E. coli
pathogen. Chronopotentiometric response of the redox indicator upon increasing the target
concentration in 1.0 mg=ml steps (a±c), in connection with a 2 min hybridization time.
(Reproduced with permission from reference 46.)
chips would integrate a micro¯uidic networkÐessential for performing all the steps
of the bioassay (see Section 6-3.2)Ðwith the detection process, and would thus
address the growing demands for shrinking DNA diagnostics in accordance with
market needs in the twenty-®rst century.
Other modes of DNA interactions (besides base-pair recognition) can be used for
the development of electrochemical DNA biosensors. In particular, dsDNA-modi®ed
electrodes can be designed for detecting small molecules (e.g., drugs or carcinogens)
interacting with the immobilized nucleic acid layer (47,48). The intercalative
accumulation of these molecules onto the surface-con®ned DNA layer can be
used for their measurements at trace levels, in a manner analogous to the
preconcentration=voltammetric schemes described in Section 4-5.3.4. In addition,
the sensitivity of the intrinsic redox signals of DNA to its structure and conformation
offers considerable opportunities for nucleic acid research, including the sensing of
DNA damage.
6-1.2.3 Receptor-Based Sensors Another new and promising avenue of
sensing is the use of chemoreceptors as biological recognition elements. Receptors
are protein molecules embedded in the cellular membrane that speci®cally bind to
target analytes. The receptor±analyte (host±guest) binding can trigger speci®c
cellular events, such as modulation of the membrane permeability or activation of
certain enzymes, that translate the chemical interaction to electrical signals. For
example, ion-channel sensors utilizing receptors in a bilayer lipid membrane couple
the speci®c binding process with intense signal ampli®cation (49±51), attributed to
the opening=closing switching of the ion ¯ux through the membrane (Figure 6-15).
A single selective binding event between the membrane receptor and the target
analyte can thus result in an increase of the transmembrane conduction that involves
thousands of ions. Unlike most antibody binding (aimed at speci®c substances),