Page 204 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 204
6-2 GAS SENSORS 189
excellent selectivity, compared with many ion-selective electrodes. Besides the
membrane, the response characteristics are often affected by the composition of
the internal solution and the geometric variables (57). Amperometric gas sensors
based on different con®gurations have also been developed in recent years. Such
developments have been reviewed (58).
6-2.1 Carbon Dioxide Sensors
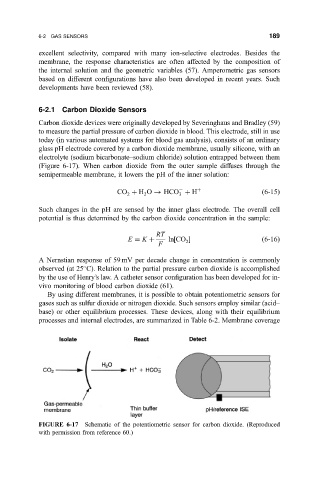
Carbon dioxide devices were originally developed by Severinghaus and Bradley (59)
to measure the partial pressure of carbon dioxide in blood. This electrode, still in use
today (in various automated systems for blood gas analysis), consists of an ordinary
glass pH electrode covered by a carbon dioxide membrane, usually silicone, with an
electrolyte (sodium bicarbonate±sodium chloride) solution entrapped between them
(Figure 6-17). When carbon dioxide from the outer sample diffuses through the
semipermeable membrane, it lowers the pH of the inner solution:
CO H O ! HCO H
6-15
2 2 3
Such changes in the pH are sensed by the inner glass electrode. The overall cell
potential is thus determined by the carbon dioxide concentration in the sample:
RT
E K lnCO
6-16
2
F
A Nernstian response of 59 mV per decade change in concentration is commonly
observed (at 25 C). Relation to the partial pressure carbon dioxide is accomplished
by the use of Henry's law. A catheter sensor con®guration has been developed for in-
vivo monitoring of blood carbon dioxide (61).
By using different membranes, it is possible to obtain potentiometric sensors for
gases such as sulfur dioxide or nitrogen dioxide. Such sensors employ similar (acid±
base) or other equilibrium processes. These devices, along with their equilibrium
processes and internal electrodes, are summarized in Table 6-2. Membrane coverage
FIGURE 6-17 Schematic of the potentiometric sensor for carbon dioxide. (Reproduced
with permission from reference 60.)