Page 203 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 203
188 ELECTROCHEMICAL SENSORS
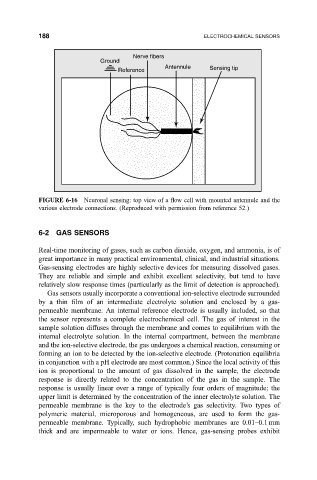
Nerve fibers
Ground
Antennule Sensing tip
Reference
FIGURE 6-16 Neuronal sensing: top view of a ¯ow cell with mounted antennule and the
various electrode connections. (Reproduced with permission from reference 52.)
6-2 GAS SENSORS
Real-time monitoring of gases, such as carbon dioxide, oxygen, and ammonia, is of
great importance in many practical environmental, clinical, and industrial situations.
Gas-sensing electrodes are highly selective devices for measuring dissolved gases.
They are reliable and simple and exhibit excellent selectivity, but tend to have
relatively slow response times (particularly as the limit of detection is approached).
Gas sensors usually incorporate a conventional ion-selective electrode surrounded
by a thin ®lm of an intermediate electrolyte solution and enclosed by a gas-
permeable membrane. An internal reference electrode is usually included, so that
the sensor represents a complete electrochemical cell. The gas of interest in the
sample solution diffuses through the membrane and comes to equilibrium with the
internal electrolyte solution. In the internal compartment, between the membrane
and the ion-selective electrode, the gas undergoes a chemical reaction, consuming or
forming an ion to be detected by the ion-selective electrode. (Protonation equilibria
in conjunction with a pH electrode are most common.) Since the local activity of this
ion is proportional to the amount of gas dissolved in the sample, the electrode
response is directly related to the concentration of the gas in the sample. The
response is usually linear over a range of typically four orders of magnitude; the
upper limit is determined by the concentration of the inner electrolyte solution. The
permeable membrane is the key to the electrode's gas selectivity. Two types of
polymeric material, microporous and homogeneous, are used to form the gas-
permeable membrane. Typically, such hydrophobic membranes are 0.01±0.1 mm
thick and are impermeable to water or ions. Hence, gas-sensing probes exhibit