Page 205 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 205
190 ELECTROCHEMICAL SENSORS
TABLE 6-2 Potentiometric Gas Sensors
Target Gas Equilibrium Process Sensing Electrode
CO H O HCO H H ; CO 2
CO 2 2 2 3 3
2NO H O NO NO 2H H
NO 2 2 2 3 2
SO H O HSO H H
SO 2 2 2 3
H 2 S H S 2H S 2 S ; H
2
2
HF HF H F F ; H
of other ion-selective electrodes (e.g., chloride) can be used for the sensing of other
gases (e.g., chlorine).
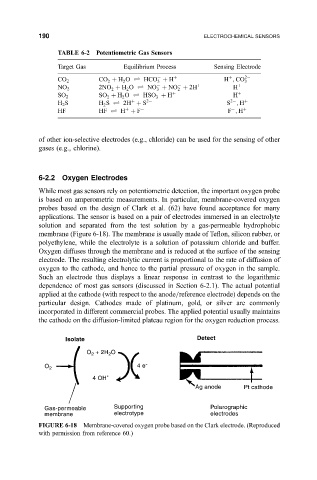
6-2.2 Oxygen Electrodes
While most gas sensors rely on potentiometric detection, the important oxygen probe
is based on amperometric measurements. In particular, membrane-covered oxygen
probes based on the design of Clark et al. (62) have found acceptance for many
applications. The sensor is based on a pair of electrodes immersed in an electrolyte
solution and separated from the test solution by a gas-permeable hydrophobic
membrane (Figure 6-18). The membrane is usually made of Te¯on, silicon rubber, or
polyethylene, while the electrolyte is a solution of potassium chloride and buffer.
Oxygen diffuses through the membrane and is reduced at the surface of the sensing
electrode. The resulting electrolytic current is proportional to the rate of diffusion of
oxygen to the cathode, and hence to the partial pressure of oxygen in the sample.
Such an electrode thus displays a linear response in contrast to the logarithmic
dependence of most gas sensors (discussed in Section 6-2.1). The actual potential
applied at the cathode (with respect to the anode=reference electrode) depends on the
particular design. Cathodes made of platinum, gold, or silver are commonly
incorporated in different commercial probes. The applied potential usually maintains
the cathode on the diffusion-limited plateau region for the oxygen reduction process.
FIGURE 6-18 Membrane-covered oxygen probe based on the Clark electrode. (Reproduced
with permission from reference 60.)