Page 207 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 207
192 ELECTROCHEMICAL SENSORS
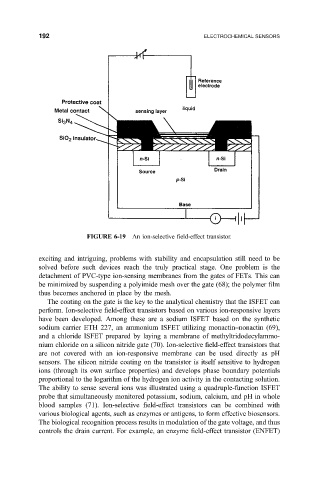
FIGURE 6-19 An ion-selective ®eld-effect transistor.
exciting and intriguing, problems with stability and encapsulation still need to be
solved before such devices reach the truly practical stage. One problem is the
detachment of PVC-type ion-sensing membranes from the gates of FETs. This can
be minimized by suspending a polyimide mesh over the gate (68); the polymer ®lm
thus becomes anchored in place by the mesh.
The coating on the gate is the key to the analytical chemistry that the ISFET can
perform. Ion-selective ®eld-effect transistors based on various ion-responsive layers
have been developed. Among these are a sodium ISFET based on the synthetic
sodium carrier ETH 227, an ammonium ISFET utilizing monactin±nonactin (69),
and a chloride ISFET prepared by laying a membrane of methyltridodecylammo-
nium chloride on a silicon nitride gate (70). Ion-selective ®eld-effect transistors that
are not covered with an ion-responsive membrane can be used directly as pH
sensors. The silicon nitride coating on the transistor is itself sensitive to hydrogen
ions (through its own surface properties) and develops phase boundary potentials
proportional to the logarithm of the hydrogen ion activity in the contacting solution.
The ability to sense several ions was illustrated using a quadruple-function ISFET
probe that simultaneously monitored potassium, sodium, calcium, and pH in whole
blood samples (71). Ion-selective ®eld-effect transistors can be combined with
various biological agents, such as enzymes or antigens, to form effective biosensors.
The biological recognition process results in modulation of the gate voltage, and thus
controls the drain current. For example, an enzyme ®eld-effect transistor (ENFET)