Page 212 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 212
6-4 SENSOR ARRAYS 197
printing technology requires lower capital and production costs than the thin-®lm
lithographic approach, but is limited to electrode structures larger than 100 mm. It is
also possible to fabricate electrochemical devices combining the thin- and thick-®lm
processes.
6-4 SENSOR ARRAYS
So far we have discussed the one-sensor=one-analyte approach. However, arrays of
independent electrodes can offer much more analytical information and thus hold a
great potential for many practical applications. These include the development of
``intelligent sensing systems'' capable of responding to changes in the chemical
environment of the array.
The use of multielectrode arrays takes advantage of the partial selectivity of an
individual electrode by combining several electrodes and examining the relative
responses of all the sensors together. The array's response for each analyte thus
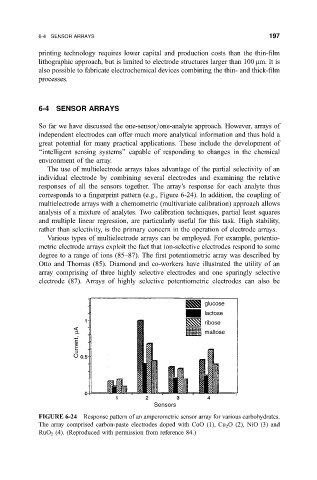
corresponds to a ®ngerprint pattern (e.g., Figure 6-24). In addition, the coupling of
multielectrode arrays with a chemometric (multivariate calibration) approach allows
analysis of a mixture of analytes. Two calibration techniques, partial least squares
and multiple linear regression, are particularly useful for this task. High stability,
rather than selectivity, is the primary concern in the operation of electrode arrays.
Various types of multielectrode arrays can be employed. For example, potentio-
metric electrode arrays exploit the fact that ion-selective electrodes respond to some
degree to a range of ions (85±87). The ®rst potentiometric array was described by
Otto and Thomas (85). Diamond and co-workers have illustrated the utility of an
array comprising of three highly selective electrodes and one sparingly selective
electrode (87). Arrays of highly selective potentiometric electrodes can also be
FIGURE 6-24 Response pattern of an amperometric sensor array for various carbohydrates.
The array comprised carbon-paste electrodes doped with CoO (1), Cu 2 O (2), NiO (3) and
RuO 2 (4). (Reproduced with permission from reference 84.)