Page 76 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 76
3-1 CHRONOAMPEROMETRY 61
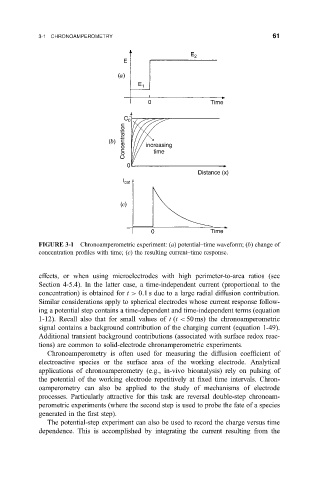
FIGURE 3-1 Chronoamperometric experiment: (a) potential±time waveform; (b) change of
concentration pro®les with time; (c) the resulting current±time response.
effects, or when using microelectrodes with high perimeter-to-area ratios (see
Section 4-5.4). In the latter case, a time-independent current (proportional to the
concentration) is obtained for t > 0:1 s due to a large radial diffusion contribution.
Similar considerations apply to spherical electrodes whose current response follow-
ing a potential step contains a time-dependent and time-independent terms (equation
1-12). Recall also that for small values of t
t < 50 ms) the chronoamperometric
signal contains a background contribution of the charging current (equation 1-49).
Additional transient background contributions (associated with surface redox reac-
tions) are common to solid-electrode chronamperometric experiments.
Chronoamperometry is often used for measuring the diffusion coef®cient of
electroactive species or the surface area of the working electrode. Analytical
applications of chronoamperometry (e.g., in-vivo bioanalysis) rely on pulsing of
the potential of the working electrode repetitively at ®xed time intervals. Chron-
oamperometry can also be applied to the study of mechanisms of electrode
processes. Particularly attractive for this task are reversal double-step chronoam-
perometric experiments (where the second step is used to probe the fate of a species
generated in the ®rst step).
The potential-step experiment can also be used to record the charge versus time
dependence. This is accomplished by integrating the current resulting from the