Page 95 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 95
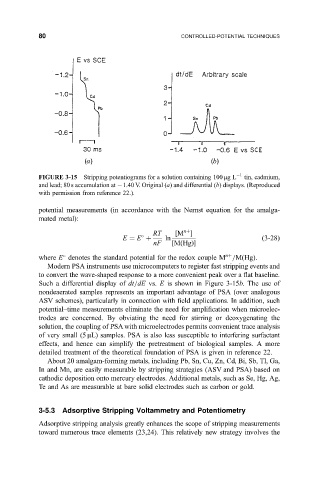
80 CONTROLLED-POTENTIAL TECHNIQUES
FIGURE 3-15 Stripping potentiograms for a solution containing 100 mgL 1 tin, cadmium,
and lead; 80 s accumulation at 1.40 V. Original (a) and differential (b) displays. (Reproduced
with permission from reference 22.).
potential measurements (in accordance with the Nernst equation for the amalga-
mated metal):
n
RT M
E E ln
3-28
nF M
Hg
n
where E denotes the standard potential for the redox couple M =M
Hg.
Modern PSA instruments use microcomputers to register fast stripping events and
to convert the wave-shaped response to a more convenient peak over a ¯at baseline.
Such a differential display of dt=dE vs. E is shown in Figure 3-15b. The use of
nondeaerated samples represents an important advantage of PSA (over analogous
ASV schemes), particularly in connection with ®eld applications. In addition, such
potential±time measurements eliminate the need for ampli®cation when microelec-
trodes are concerned. By obviating the need for stirring or deoxygenating the
solution, the coupling of PSA with microelectrodes permits convenient trace analysis
of very small (5 mL) samples. PSA is also less susceptible to interfering surfactant
effects, and hence can simplify the pretreatment of biological samples. A more
detailed treatment of the theoretical foundation of PSA is given in reference 22.
About 20 amalgam-forming metals, including Pb, Sn, Cu, Zn, Cd, Bi, Sb, Tl, Ga,
In and Mn, are easily measurable by stripping strategies (ASV and PSA) based on
cathodic deposition onto mercury electrodes. Additional metals, such as Se, Hg, Ag,
Te and As are measurable at bare solid electrodes such as carbon or gold.
3-5.3 Adsorptive Stripping Voltammetry and Potentiometry
Adsorptive stripping analysis greatly enhances the scope of stripping measurements
toward numerous trace elements (23,24). This relatively new strategy involves the