Page 96 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 96
3-5 STRIPPING ANALYSIS 81
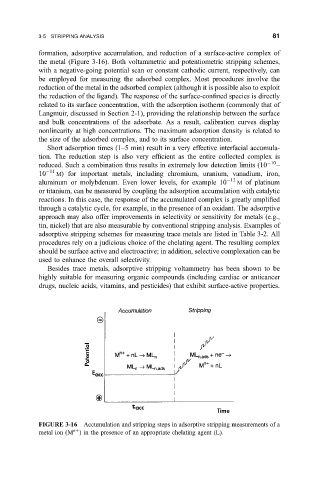
formation, adsorptive accumulation, and reduction of a surface-active complex of
the metal (Figure 3-16). Both voltammetric and potentiometric stripping schemes,
with a negative-going potential scan or constant cathodic current, respectively, can
be employed for measuring the adsorbed complex. Most procedures involve the
reduction of the metal in the adsorbed complex (although it is possible also to exploit
the reduction of the ligand). The response of the surface-con®ned species is directly
related to its surface concentration, with the adsorption isotherm (commonly that of
Langmuir, discussed in Section 2-1), providing the relationship between the surface
and bulk concentrations of the adsorbate. As a result, calibration curves display
nonlinearity at high concentrations. The maximum adsorption density is related to
the size of the adsorbed complex, and to its surface concentration.
Short adsorption times (1±5 min) result in a very effective interfacial accumula-
tion. The reduction step is also very ef®cient as the entire collected complex is
reduced. Such a combination thus results in extremely low detection limits (10 10 ±
10 11 M) for important metals, including chromium, uranium, vanadium, iron,
aluminum or molybdenum. Even lower levels, for example 10 12 M of platinum
or titanium, can be measured by coupling the adsorption accumulation with catalytic
reactions. In this case, the response of the accumulated complex is greatly ampli®ed
through a catalytic cycle, for example, in the presence of an oxidant. The adsorptive
approach may also offer improvements in selectivity or sensitivity for metals (e.g.,
tin, nickel) that are also measurable by conventional stripping analysis. Examples of
adsorptive stripping schemes for measuring trace metals are listed in Table 3-2. All
procedures rely on a judicious choice of the chelating agent. The resulting complex
should be surface active and electroactive; in addition, selective complexation can be
used to enhance the overall selectivity.
Besides trace metals, adsorptive stripping voltammetry has been shown to be
highly suitable for measuring organic compounds (including cardiac or anticancer
drugs, nucleic acids, vitamins, and pesticides) that exhibit surface-active properties.
FIGURE 3-16 Accumulation and stripping steps in adsorptive stripping measurements of a
n
metal ion
M in the presence of an appropriate chelating agent (L).