Page 380 - Applied Process Design For Chemical And Petrochemical Plants Volume III
P. 380
66131_Ludwig_CH11A 5/30/2001 4:50 PM Page 337
Refrigeration Systems 337
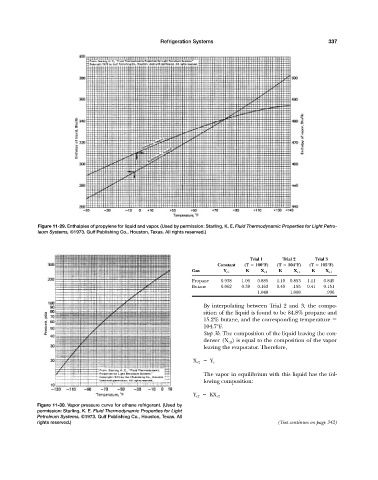
Figure 11-29. Enthalpies of propylene for liquid and vapor. (Used by permission: Starling, K. E. Fluid Thermodynamic Properties for Light Petro-
leum Systems, ©1973. Gulf Publishing Co., Houston, Texas. All rights reserved.)
Trial 1 Trial 2 Trial 3
Constant (T 100°F) (T 104°F) (T 105°F)
Gas Y c l K X c l K X c l K X c l
Propane 0.938 1.06 0.885 1.10 0.853 1.11 0.845
Butane 0.062 0.38 0.163 0.40 .155 0.41 0.151
1.048 1.008 .996
By interpolating between Trial 2 and 3, the compo-
sition of the liquid is found to be 84.8% propane and
15.2% butane, and the corresponding temperature
104.7°F.
Step 3b. The composition of the liquid leaving the con-
denser (X c2 ) is equal to the composition of the vapor
leaving the evaporator. Therefore,
X c2 Y e
The vapor in equilibrium with this liquid has the fol-
lowing composition:
Y c2 KX c2
Figure 11-30. Vapor pressure curve for ethane refrigerant. (Used by
permission: Starling, K. E. Fluid Thermodynamic Properties for Light
Petroleum Systems, ©1973. Gulf Publishing Co., Houston, Texas. All
rights reserved.) (Text continues on page 342)