Page 379 - Applied Process Design For Chemical And Petrochemical Plants Volume III
P. 379
66131_Ludwig_CH11A 5/30/2001 4:50 PM Page 336
336 Applied Process Design for Chemical and Petrochemical Plants
corresponding pressure in the evaporator is therefore
fixed. Because the sum of all the Y e mol fractions must
add up to 1,000, assume pressures until the Y e 1,000
to determine the correct pressure.
Trial 1 Trial 2 Trial 3
Constant (p 30 psia) (p 35 psia) (p 32 psia)
Gas
X e K Y e K Y e K Y e
Propane 0.75 1.32 0.990 1.15 0.863 1.25 0.938
Butane 0.25 0.263 0.066 0.23 0.0575 0.25 0.062
1.056 0.9205 1.000
Therefore the pressure in the evaporator is 32 psia, and
the vapor composition is 93.8% propane and 6.2%
butane. This is the vapor composition that the
compressor must handle (if the original assumption
holds true).
Step 3a. The calculations to determine condenser pres-
sure are handled somewhat similarly to those of the
evaporator except that they must be divided into two
parts.
The vapor composition at the top of the condenser (Y c1 )
is different from that at the bottom (Y c2 ). The condenser
may be compared to a fractional distillation problem in
reverse. Butane, having a higher boiling point, will con-
dense out faster than the propane, although both are
condensing at the same time. Thus, the vapor and liquid
mol fractions from the top to the bottom of the con-
denser tube bundle are always changing. Proceed as fol-
lows: The vapor at the top has the same composition as
the gas leaving the evaporator. Therefore, Y c1 Y e .
The composition of the liquid in equilibrium with this
vapor is calculated from
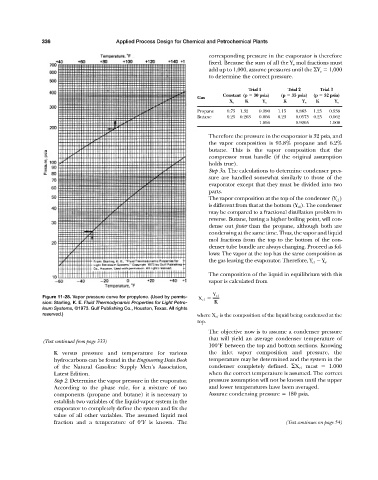
Figure 11-28. Vapor pressure curve for propylene. (Used by permis- X c1 Y c1
sion: Starling, K. E. Fluid Thermodynamic Properties for Light Petro- K
leum Systems, ©1973. Gulf Publishing Co., Houston, Texas. All rights
reserved.) where X c1 is the composition of the liquid being condensed at the
top.
The objective now is to assume a condenser pressure
that will yield an average condenser temperature of
(Text continued from page 333)
100°F between the top and bottom sections. Knowing
K versus pressure and temperature for various the inlet vapor composition and pressure, the
hydrocarbons can be found in the Engineering Data Book temperature may be determined and the system in the
of the Natural Gasoline Supply Men’s Association, condenser completely defined. X c1 must 1.000
Latest Edition. when the correct temperature is assumed. The correct
Step 2. Determine the vapor pressure in the evaporator. pressure assumption will not be known until the upper
According to the phase rule, for a mixture of two and lower temperatures have been averaged.
components (propane and butane) it is necessary to Assume condensing pressure 180 psia.
establish two variables of the liquid-vapor system in the
evaporator to completely define the system and fix the
value of all other variables. The assumed liquid mol
fraction and a temperature of 0°F is known. The (Text continues on page 54)