Page 376 - Applied Process Design For Chemical And Petrochemical Plants Volume III
P. 376
66131_Ludwig_CH11A 5/30/2001 4:50 PM Page 333
Refrigeration Systems 333
the evaporator has changed, and the composition of the
vapor in equilibrium with it is not known. Vapor and liquid
compositions in the condenser are also not known, but
three important facts can be established:
1. By material balance, the composition of the vapor
entering the condenser is the same composition as the
liquid leaving the condenser (with no bleed off).
2. The condensed liquid at the top of the condenser is in
equilibrium with the vapor composition entering the
condenser, which is also the composition of the vapor
leaving the evaporator.
3. The mixture (liquid and vapor) in the total system must
have the same overall composition as the initial charge.
The problem is one of trial and error and illustrates the
procedure.
Liquid and Vapor Equilibrium
See Chapter 12 for Mollier Diagrams.
Given
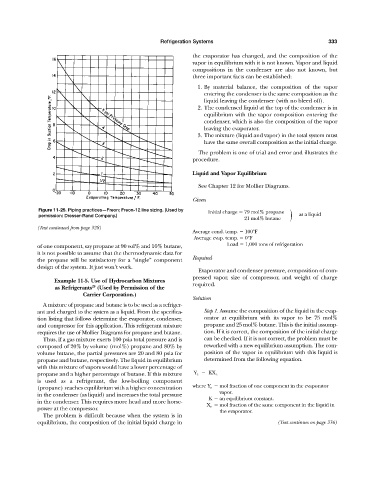
Figure 11-25. Piping practices—Freon; Freon-12 line sizing. (Used by Initial charge 79 mol% propane
permission: Dresser-Rand Company.) 21 mol% butane as a liquid
(Text continued from page 328)
Average cond. temp. 100°F
Average evap. temp. 0°F
of one component, say propane at 90 vol% and 10% butane, Load 1,000 tons of refrigeration
it is not possible to assume that the thermodynamic data for
Required
the propane will be satisfactory for a “single” component
design of the system. It just won’t work.
Evaporator and condenser pressure, composition of com-
pressed vapor, size of compressor, and weight of charge
Example 11-5. Use of Hydrocarbon Mixtures
required.
29
as Refrigerants (Used by Permission of the
Carrier Corporation.)
Solution
A mixture of propane and butane is to be used as a refriger-
ant and charged to the system as a liquid. From the specifica- Step 1. Assume the composition of the liquid in the evap-
tion listing that follows determine the evaporator, condenser, orator at equilibrium with its vapor to be 75 mol%
and compressor for this application. This refrigerant mixture propane and 25 mol% butane. This is the initial assump-
requires the use of Mollier Diagrams for propane and butane. tion. If it is correct, the composition of the initial charge
Thus, if a gas mixture exerts 100 psia total pressure and is can be checked. If it is not correct, the problem must be
composed of 20% by volume (mol%) propane and 80% by reworked with a new equilibrium assumption. The com-
volume butane, the partial pressures are 20 and 80 psia for position of the vapor in equilibrium with this liquid is
propane and butane, respectively. The liquid in equilibrium determined from the following equation.
with this mixture of vapors would have a lower percentage of
propane and a higher percentage of butane. If this mixture Y e KX e
is used as a refrigerant, the low-boiling component
(propane) reaches equilibrium with a higher concentration where Y e mol fraction of one component in the evaporator
vapor.
in the condenser (as liquid) and increases the total pressure
K an equilibrium constant.
in the condenser. This requires more head and more horse-
X e mol fraction of the same component in the liquid in
power at the compressor.
the evaporator.
The problem is difficult because when the system is in
equilibrium, the composition of the initial liquid charge in (Text continues on page 336)