Page 372 - Applied Process Design For Chemical And Petrochemical Plants Volume III
P. 372
66131_Ludwig_CH11A 5/30/2001 4:50 PM Page 329
Refrigeration Systems 329
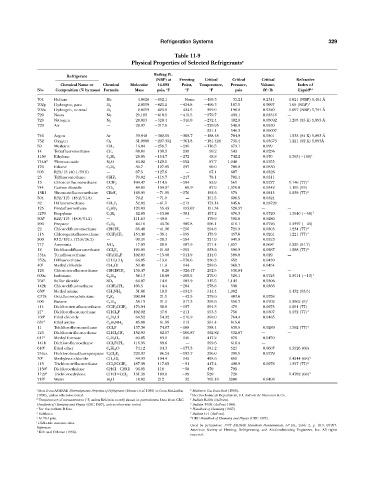
Table 11-9
Physical Properties of Selected Refrigerants a
Boiling Pt.
Refrigerant
(NBP) at Freezing Critical Critical Critical Refractive
Chemical Name or Chemical Molecular 14.693 Point, Temperature, Pressure, Volume, Index of
3
o
No. Composition (% by mass) Formula Mass psia, F o F o F psia ft /lb Liquid b,c
704 Helium He 4.0026 452.1 None 450.3 33.21 0.2311 1.021 (NBP) 5,461 Å
702p Hydrogen, para H 2 2.0159 423.2 434.8 400.3 187.5 0.5097 1.09 (NBP) f
702n Hydrogen, normal H 2 2.0159 423.0 434.5 399.9 190.8 0.5320 1.097 (NBP) 5,791 Å
720 Neon Ne 20.183 410.9 415.5 379.7 493.1 0.03316 —
728 Nitrogen N 2 28.013 320.4 346.0 232.4 492.9 0.05092 1.205 (83 K) 5,893 Å
729 Air — 28.97 317.8 — 220.95 548.9 0.0530
221.1 546.3 0.05007
740 Argon Ar 39.948 302.55 308.7 188.48 704.9 0.0301 1.233 (84 K) 5,893 Å
732 Oxygen O 2 31.9988 297.332 361.8 181.424 731.4 0.03673 1.221 (92 K) 5,893Å
50 Methane CH 4 16.04 258.7 296 116.5 673.1 0.099
14 Tetrafluoromethane CF 4 88.01 198.3 299 50.2 543 0.0256
1150 Ethylene C 2 H 4 28.05 154.7 272 48.8 742.2 0.070 1.363( 148) l
744A 2 2 Nitrous oxide N 2 O 44.02 129.1 152 97.7 1,048 0.0355
170 Ethane C 2 H 6 30.7 127.85 297 90.0 709.8 0.0830
503 R23/13 (40.1/59.9) — 87.5 127.6 — 67.1 607 0.0326
23 Trifluoromethane CHF 3 70.02 115.7 247 78.1 701.4 0.0311
13 Chlorotrifluoromethane CClF 3 104.47 114.6 294 83.9 561 0.0277 1.146 (77) 4
744 Carbon dioxide CO 2 44.01 109.2 d 69.9 c 87.9 1,070.0 0.0342 1.195 (59)
13B1 1 Bromotrifluoromethane CBrF 3 148.93 71.95 270 152.6 575 0.0215 1.239 (77) 4
504 R32/115 (48.2/51.8) — 79.2 71.0 — 151.5 690.5 0.0324
32 Difluoromethane CH 2 F 2 52.02 61.1 213 173.14 845.6 0.03726
125 Pentafluoroethane C 2 HF 5 120.03 55.43 153.67 151.34 526.57 — —
1270 Propylene C 3 H 6 42.09 53.86 301 197.2 670.3 0.0720 1.3640 ( 58) 1
502 5 R22/115 (48.8/51.2) — 111.63 49.8 — 179.9 591.0 0.0286
290 Propane C 3 H 8 44.10 43.76 305.8 206.1 616.1 0.0726 1.3397 ( 43)
22 Chlorodifluoromethane CHClF 2 86.48 41.36 256 204.8 721.9 0.0305 1.234 (77) 4
115 Chloropentafluoroethane CClF 2 CF 3 154.48 38.4 159 175.9 457.6 0.0261 1.221 (77) 4
500 R12/152 a (73.8/26.2) — 99.31 28.3 254 221.9 641.9 0.0323
717 Ammonia NH 3 17.03 28.0 107.9 271.4 1,657 0.068 d 1.325 (61.7)
12 Dichlorodifluoromethane CCl 2 F 2 120.93 21.62 252 233.6 596.9 0.0287 1.288 (77) 4
134a Tetrafluoroethane CF 3 CH 2 F 102.03 15.08 141.9 214.0 589.8 0.029 —
152a Difluoroethane CHF 2 CH 3 66.05 13.0 178.6 236.3 652 0.0439
40 2 Methyl chloride CH 3 Cl 50.49 11.6 144 289.6 968.7 0.0454
124 Chlorotetrafluoroethane CHClFCF 3 136.47 8.26 326.47 252.5 530.84 — —
600a Isobutane C 4 H 10 58.13 10.89 255.5 275.0 529.1 0.0725 1.3514 ( 13) 1
764 6 Sulfur dioxide SO 2 64.07 14.0 103.9 315.5 1,143 0.0306
142b Chlorodifluoroethane CClF 2 CH 3 100.5 14.4 204 278.8 598 0.0368
630 6 Methyl amine CH 3 NH 2 31.06 19.9 134.5 314.4 1,082 1.432 (63.5)
C318 Octafluorocyclobutane C 4 F 8 200.04 21.5 42.5 239.6 403.6 0.0258
600 Butane C 4 H 10 58.13 31.1 217.3 305.6 550.7 0.0702 1.3562 (5) 1
114 Dichlorotetrafluoroethane CClF 2 CClF 2 170.94 38.8 137 294.3 473 0.0275 1.294 (77)
21 7 Dichlorofluoromethane CHCl 2 F 102.92 47.8 211 353.3 750 0.0307 1.332 (77) 4
160 2 Ethyl chloride C 2 H 5 Cl 64.52 54.32 216.9 369.0 764.4 0.0485
631 6 Ethyl amine C 2 H 5 NH 2 45.08 61.88 113 361.4 815.6
11 Trichlorofluoromethane CCl 3 F 137.38 74.87 168 388.4 639.5 0.0289 1.362 (77) 4
123 Dichlorotrifluoroethane CHCl 2 CF 3 152.93 82.17 160.87 362.82 532.87 — —
611 6 Methyl formate C 2 H 4 O 2 60.05 89.2 146 417.2 870 0.0459
141b Dichlorofluoroethane CCl 2 FCH 3 116.95 89.6 — 399.6 616.4 —
610 6 Ethyl ether C 4 H 10 O 74.12 94.3 177.3 381.2 523 0.0607 1.3526 (68)
216ca Dichlorohexafluoropropane C 3 Cl 2 F 6 220.93 96.24 193.7 356.0 399.5 0.0279
30 6 Methylene chloride CH 2 Cl 2 84.93 104.4 142 458.6 882 1.4244 (68) 3
113 Trichlorotrifluoroethane CCl 2 FCClF 2 187.39 117.63 31 417.4 498.9 0.0278 1.357 (77) 4
1130 8 Dichloroethylene CHCl CHCl 96.95 118 58 470 795
1120 6 Trichloroethylene CHCl CCl 2 131.39 189.0 99 520 728 1.4782 (68) 3
718 6 Water H 2 O 18.02 212 32 705.18 3200 0.0498
a Data from ASHRAE Thermodynamic Properties of Refrigerants (Stewart et al. 1986) or from McLinden 2 Matheson Gas Data Book (1966).
(1990), unless otherwise noted. 3 Electrochemicals Department, E.I. duPont de Nemours & Co.
b Temperature of measurement (°F, unless Kelvin is noted) shown in parentheses. Data from CRC 4 Bulletin B-32A (duPont).
Handbook of Chemistry and Physics (CRC 1987), unless otherwise noted. 5 Bulletin T-502 (duPont 1980).
c For the sodium D line. 6 Handbook of Chemistry (1967).
d Sublimes. 7 Bulletin G-1 (duPont).
e At 76.4 psia. 8 CRC Handbook of Chemistry and Physics (CRC 1987).
f Dielectric constant data.
Used by permission: 1997 ASHRAE Handbook Fundamentals, I-P Ed., Table 2, p. 18.3, ©1997.
References:
American Society of Heating, Refrigerating, and Air-Conditioning Engineers, Inc. All rights
1 Kirk and Othmer (1956).
reserved.