Page 25 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 25
1.2 WHAT MAKES FOR A GOOD NUCLEOPHILE? 5
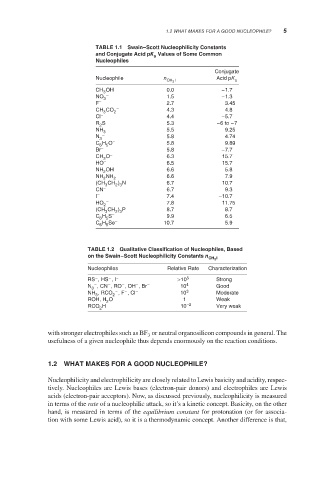
TABLE 1.1 Swain–Scott Nucleophilicity Constants
and Conjugate Acid pK Values of Some Common
a
Nucleophiles
Conjugate
Nucleophile n CH 3 I Acid pK a
CH OH 0.0 −1.7
3
NO 3 – 1.5 −1.3
F – 2.7 3.45
CH CO 2 – 4.3 4.8
3
Cl – 4.4 −5.7
R S 5.3 −6to −7
2
NH 3 5.5 9.25
−
N 5.8 4.74
3
C H O – 5.8 9.89
6 5
Br – 5.8 −7.7
CH O – 6.3 15.7
3
HO – 6.5 15.7
NH OH 6.6 5.8
2
NH NH 2 6.6 7.9
2
(CH CH ) N 6.7 10.7
2 3
3
CN – 6.7 9.3
I – 7.4 −10.7
HO 2 – 7.8 11.75
(CH CH ) P 8.7 8.7
3
2 3
C H S – 9.9 6.5
6
5
C H Se − 10.7 5.9
5
6
TABLE 1.2 Qualitative Classification of Nucleophiles, Based
CH 3 I
on the Swain–Scott Nucleophilicity Constants n
Nucleophiles Relative Rate Characterization
−
−
RS ,HS ,I − >10 5 Strong
−
−
−
N 3 − ,CN ,RO ,OH ,Br − 10 4 Good
−
NH , RCO 2 − ,F ,Cl − 10 3 Moderate
3
ROH, H O 1 Weak
2
RCO H 10 −2 Very weak
2
with stronger electrophiles such as BF or neutral organosilicon compounds in general. The
3
usefulness of a given nucleophile thus depends enormously on the reaction conditions.
1.2 WHAT MAKES FOR A GOOD NUCLEOPHILE?
Nucleophilicity and electrophilicity are closely related to Lewis basicity and acidity, respec-
tively. Nucleophiles are Lewis bases (electron-pair donors) and electrophiles are Lewis
acids (electron-pair acceptors). Now, as discussed previously, nucleophilicity is measured
in terms of the rate of a nucleophilic attack, so it’s a kinetic concept. Basicity, on the other
hand, is measured in terms of the equilibrium constant for protonation (or for associa-
tion with some Lewis acid), so it is a thermodynamic concept. Another difference is that,