Page 30 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 30
A COLLECTION OF BASIC CONCEPTS
10
Fluoride and cyanide are very much worse leaving groups than the pK values of HF
a
and HCN would imply. This presumably reflects the great strength of the C–F and C–CN
bonds.
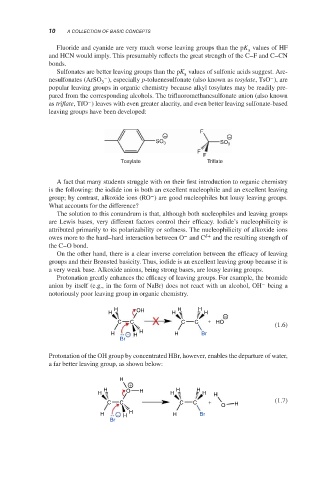
Sulfonates are better leaving groups than the pK values of sulfonic acids suggest. Are-
a
−
−
nesulfonates (ArSO ), especially p-toluenesulfonate (also known as tosylate,TsO ), are
3
popular leaving groups in organic chemistry because alkyl tosylates may be readily pre-
pared from the corresponding alcohols. The trifluoromethanesulfonate anion (also known
−
as triflate,TfO ) leaves with even greater alacrity, and even better leaving sulfonate-based
leaving groups have been developed:
F
− −
SO 3 SO 3
F
F
Tosylate Triflate
A fact that many students struggle with on their first introduction to organic chemistry
is the following: the iodide ion is both an excellent nucleophile and an excellent leaving
−
group; by contrast, alkoxide ions (RO ) are good nucleophiles but lousy leaving groups.
What accounts for the difference?
The solution to this conundrum is that, although both nucleophiles and leaving groups
are Lewis bases, very different factors control their efficacy. Iodide’s nucleophilicity is
attributed primarily to its polarizability or softness. The nucleophilicity of alkoxide ions
− +
owes more to the hard–hard interaction between O and C and the resulting strength of
the C–O bond.
On the other hand, there is a clear inverse correlation between the efficacy of leaving
groups and their Brønsted basicity. Thus, iodide is an excellent leaving group because it is
a very weak base. Alkoxide anions, being strong bases, are lousy leaving groups.
Protonation greatly enhances the efficacy of leaving groups. For example, the bromide
−
anion by itself (e.g., in the form of NaBr) does not react with an alcohol, OH being a
notoriously poor leaving group in organic chemistry.
H OH H H
H H H
−
C C C C C + HO
(1.6)
H − H H H Br
Br
Protonation of the OH group by concentrated HBr, however, enables the departure of water,
a far better leaving group, as shown below:
H
+
H O H H H
H H H H
C C C C + H (1.7)
O
H − H H H Br
Br