Page 35 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 35
1.8 PROTON TRANSFERS (PTs) 15
An E2 elimination is often the favored pathway in such cases. Similarly, a strong,
sterically hindered base also tends to favor an E2 over an S 2 pathway. A good example
N
is potassium t-butoxide, which is used in reaction 1.8. Examples of sterically hindered
nitrogen bases include N,N-diisopropylethylamine (DIPEA, also known as Hünig’s
base) and the much stronger lithium diisopropylamide (LDA). The pK of the conjugate
a
acid of DIPEA is about 9.0. Amidines, which are all-nitrogen analogs of carboxylic
acids, tend to be significantly more basic than tertiary amines. Two moderately ster-
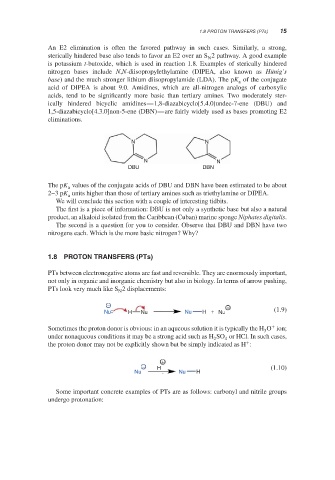
ically hindered bicyclic amidines—1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and
1,5-diazabicyclo[4.3.0]non-5-ene (DBN)—are fairly widely used as bases promoting E2
eliminations.
N N
N N
DBU DBN
The pK values of the conjugate acids of DBU and DBN have been estimated to be about
a
2–3 pK units higher than those of tertiary amines such as triethylamine or DIPEA.
a
We will conclude this section with a couple of interesting tidbits.
The first is a piece of information: DBU is not only a synthetic base but also a natural
product, an alkaloid isolated from the Caribbean (Cuban) marine sponge Niphates digitalis.
The second is a question for you to consider. Observe that DBU and DBN have two
nitrogens each. Which is the more basic nitrogen? Why?
1.8 PROTON TRANSFERS (PTs)
PTs between electronegative atoms are fast and reversible. They are enormously important,
not only in organic and inorganic chemistry but also in biology. In terms of arrow pushing,
PTs look very much like S 2 displacements:
N
− −
Nu H Nu Nu H + Nu (1.9)
+
Sometimes the proton donor is obvious: in an aqueous solution it is typically the H O ion;
3
under nonaqueous conditions it may be a strong acid such as H SO or HCl. In such cases,
4
2
+
the proton donor may not be explicitly shown but be simply indicated as H :
+
− H (1.10)
Nu Nu H
Some important concrete examples of PTs are as follows: carbonyl and nitrile groups
undergo protonation: