Page 40 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 40
A COLLECTION OF BASIC CONCEPTS
20
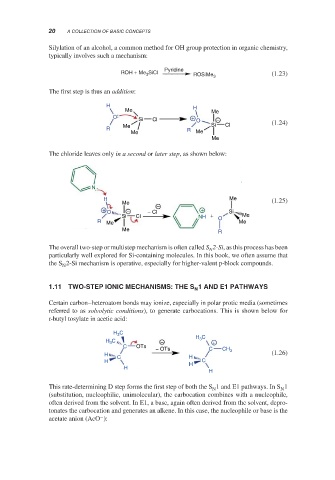
Silylation of an alcohol, a common method for OH group protection in organic chemistry,
typically involves such a mechanism:
Pyridine
ROH + Me 3 SiCl
ROSiMe 3 (1.23)
The first step is thus an addition:
H H
Me Me
O +
Si Cl O −
Me Si Cl (1.24)
R R
Me Me
Me
The chloride leaves only in a second or later step, as shown below:
N
H Me (1.25)
Me −
+ O − − Cl + Si
Si Cl NH + O Me
R Me Me
Me
R
The overall two-step or multistep mechanism is often called S 2-Si, as this process has been
N
particularly well explored for Si-containing molecules. In this book, we often assume that
the S 2-Si mechanism is operative, especially for higher-valent p-block compounds.
N
1.11 TWO-STEP IONIC MECHANISMS: THE S 1 AND E1 PATHWAYS
N
Certain carbon–heteroatom bonds may ionize, especially in polar protic media (sometimes
referred to as solvolytic conditions), to generate carbocations. This is shown below for
t-butyl tosylate in acetic acid:
H C
3
H 3 C
H 3 C − +
C OTs
− OTs C CH 3
H (1.26)
C H
H H C
H
H
This rate-determining D step forms the first step of both the S 1 and E1 pathways. In S 1
N N
(substitution, nucleophilic, unimolecular), the carbocation combines with a nucleophile,
often derived from the solvent. In E1, a base, again often derived from the solvent, depro-
tonates the carbocation and generates an alkene. In this case, the nucleophile or base is the
−
acetate anion (AcO ):