Page 43 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 43
1.13 ELECTROPHILIC SUBSTITUTION ON AROMATICS: ADDITION–ELIMINATION 23
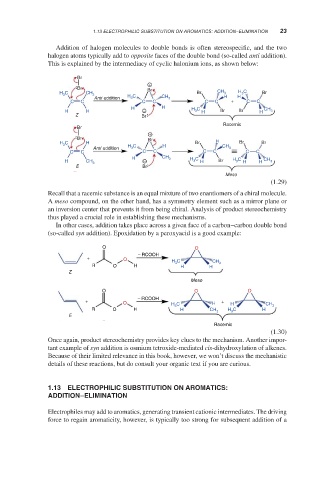
Addition of halogen molecules to double bonds is often stereospecific, and the two
halogen atoms typically add to opposite faces of the double bond (so-called anti addition).
This is explained by the intermediacy of cyclic halonium ions, as shown below:
Br
+
Br Br
H 3 C CH 3 Br CH 3 H 3 C Br
Anti addition H 3 C CH 3 H H
C C C C C C + C C
H H C CH
H H − H 3 H Br Br H 3
Z Br
Racemic
Br
+
Br Br
C H Br H Br Br
H 3 C H CH
Anti addition H 3 3
C C C C C C C C
H CH 3 C H 3 C
H CH 3 − H 3 H Br H H CH 3
E Br
Meso
(1.29)
Recall that a racemic substance is an equal mixture of two enantiomers of a chiral molecule.
A meso compound, on the other hand, has a symmetry element such as a mirror plane or
an inversion center that prevents it from being chiral. Analysis of product stereochemistry
thus played a crucial role in establishing these mechanisms.
In other cases, addition takes place across a given face of a carbon–carbon double bond
(so-called syn addition). Epoxidation by a peroxyacid is a good example:
O O
− RCOOH
+ O
H C CH
3
R O H H H 3
Z
Meso
O O O
+ − RCOOH +
O H C H H CH
3 3
R O H H CH 3 H C H
3
E
Racemic
(1.30)
Once again, product stereochemistry provides key clues to the mechanism. Another impor-
tant example of syn addition is osmium tetroxide-mediated cis-dihydroxylation of alkenes.
Because of their limited relevance in this book, however, we won’t discuss the mechanistic
details of these reactions, but do consult your organic text if you are curious.
1.13 ELECTROPHILIC SUBSTITUTION ON AROMATICS:
ADDITION–ELIMINATION
Electrophiles may add to aromatics, generating transient cationic intermediates. The driving
force to regain aromaticity, however, is typically too strong for subsequent addition of a