Page 44 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 44
A COLLECTION OF BASIC CONCEPTS
24
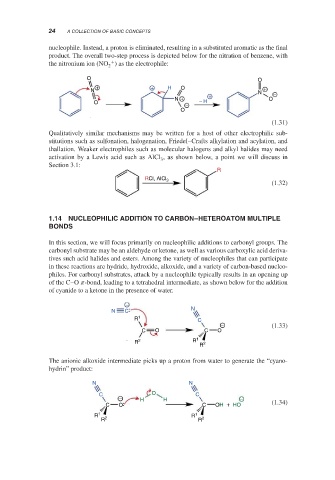
nucleophile. Instead, a proton is eliminated, resulting in a substituted aromatic as the final
product. The overall two-step process is depicted below for the nitration of benzene, with
+
the nitronium ion (NO ) as the electrophile:
2
O O
+ + H O
N N + −
N + + O
O − H
−
O
(1.31)
Qualitatively similar mechanisms may be written for a host of other electrophilic sub-
stitutions such as sulfonation, halogenation, Friedel–Crafts alkylation and acylation, and
thallation. Weaker electrophiles such as molecular halogens and alkyl halides may need
activation by a Lewis acid such as AlCl , as shown below, a point we will discuss in
3
Section 3.1:
R
RCl, AlCl 3
(1.32)
NUCLEOPHILIC ADDITION TO CARBON–HETEROATOM MULTIPLE
1.14
BONDS
In this section, we will focus primarily on nucleophilic additions to carbonyl groups. The
carbonyl substrate may be an aldehyde or ketone, as well as various carboxylic acid deriva-
tives such acid halides and esters. Among the variety of nucleophiles that can participate
in these reactions are hydride, hydroxide, alkoxide, and a variety of carbon-based nucleo-
philes. For carbonyl substrates, attack by a nucleophile typically results in an opening up
of the C–O -bond, leading to a tetrahedral intermediate, as shown below for the addition
of cyanide to a ketone in the presence of water.
−
N C N
R 1 C
− (1.33)
C O C O
R 2 R 1 2
R
The anionic alkoxide intermediate picks up a proton from water to generate the “cyano-
hydrin” product:
N N
C O C
− H H −
C O C OH + HO (1.34)
R 1 R 1
R 2 R 2