Page 48 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 48
A COLLECTION OF BASIC CONCEPTS
28
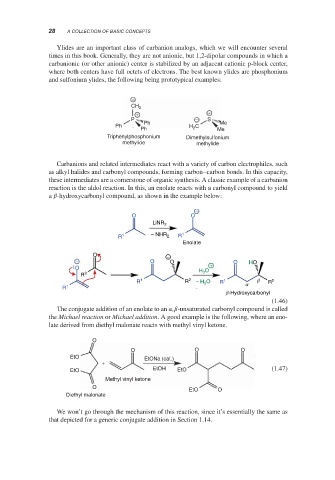
Ylides are an important class of carbanion analogs, which we will encounter several
times in this book. Generally, they are not anionic, but 1,2-dipolar compounds in which a
carbanionic (or other anionic) center is stabilized by an adjacent cationic p-block center,
where both centers have full octets of electrons. The best known ylides are phosphonium
and sulfonium ylides, the following being prototypical examples:
−
CH 2
+ +
P − S
Ph Ph H C Me
Ph 2 Me
Triphenylphosphonium Dimethylsulfonium
methylide methylide
Carbanions and related intermediates react with a variety of carbon electrophiles, such
as alkyl halides and carbonyl compounds, forming carbon–carbon bonds. In this capacity,
these intermediates are a cornerstone of organic synthesis. A classic example of a carbanion
reaction is the aldol reaction. In this, an enolate reacts with a carbonyl compound to yield
a -hydroxycarbonyl compound, as shown in the example below:
−
O O
LiNR 2
R 1 − NHR 2 R 1
Enolate
O −
− O O O HO
O H O +
R 2 3
R 1 R 2 − H O R 1 α β R 2
2
R 1
β-Hydroxycarbonyl
(1.46)
The conjugate addition of an enolate to an , -unsaturated carbonyl compound is called
the Michael reaction or Michael addition. A good example is the following, where an eno-
late derived from diethyl malonate reacts with methyl vinyl ketone.
O
O O O
EtO EtONa (cat.)
+
EtO EtOH EtO (1.47)
Methyl vinyl ketone
O
EtO O
Diethyl malonate
We won’t go through the mechanism of this reaction, since it’s essentially the same as
that depicted for a generic conjugate addition in Section 1.14.