Page 50 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 50
A COLLECTION OF BASIC CONCEPTS
30
H
− −
Base − Cl
(b) C C Cl C
Cl Cl Cl Cl Cl
Cl Cl
(1.49)
+ NaH
(c) N N N N
R R − H 2 R R
H
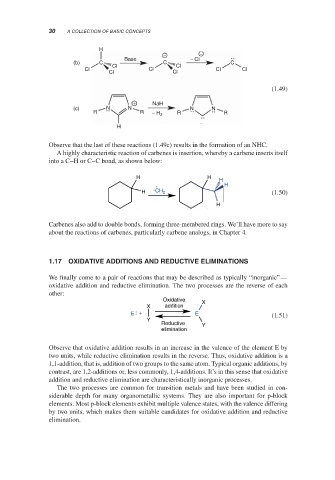
Observe that the last of these reactions (1.49c) results in the formation of an NHC.
A highly characteristic reaction of carbenes is insertion, whereby a carbene inserts itself
into a C–H or C–C bond, as shown below:
H H
H
H
H CH 2 (1.50)
H
Carbenes also add to double bonds, forming three-membered rings. We’ll have more to say
about the reactions of carbenes, particularly carbene analogs, in Chapter 4.
1.17 OXIDATIVE ADDITIONS AND REDUCTIVE ELIMINATIONS
We finally come to a pair of reactions that may be described as typically “inorganic”—
oxidative addition and reductive elimination. The two processes are the reverse of each
other:
Oxidative X
X addition
E + E (1.51)
Y
Reductive Y
elimination
Observe that oxidative addition results in an increase in the valence of the element E by
two units, while reductive elimination results in the reverse. Thus, oxidative addition is a
1,1-addition, that is, addition of two groups to the same atom. Typical organic additions, by
contrast, are 1,2-additions or, less commonly, 1,4-additions. It’s in this sense that oxidative
addition and reductive elimination are characteristically inorganic processes.
The two processes are common for transition metals and have been studied in con-
siderable depth for many organometallic systems. They are also important for p-block
elements. Most p-block elements exhibit multiple valence states, with the valence differing
by two units, which makes them suitable candidates for oxidative addition and reductive
elimination.