Page 53 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 53
1.19 LIGAND EXCHANGE REACTIONS 33
A standard method of work-up for organoboranes obtained with the hydroboration reac-
tion involves reaction with alkaline hydrogen peroxide. Mechanistically, the first step is an
A reaction between the organoborane and a hydroperoxide anion that is present in solution:
R R
O − −
H O B O B R (1.61)
R R H O R
The anionic boron center then acts as a launchpad for a migrating R group:
R R OR
− − −
− − OH HOO HOO
O B B B (1.62)
H O R
R RO R RO OR
Note that the leaving group here is hydroxide, normally a lousy one in organic chemistry.
Here, however, the electrophilic site is an oxygen and an O–O bond is a weak one that
is easily cleaved. We will encounter several examples of similar 1,2-shifts as we progress
through the book.
1.19 LIGAND EXCHANGE REACTIONS
Main-group element chemistry is replete with ligand exchange reactions or metatheses. We
will encounter a fair number of such reactions in this book, a few examples being as follows:
SnCl + SnR → 2 SnR Cl (1.63)
4 4 2 2
SO + PCl → SOCl + POCl (1.64)
2 5 2 3
2 (CH ) SiBr + SeCl → 2 (CH ) SiCl + SeBr (1.65)
3 3 2 3 3 2
IF + POF → IOF + PF (1.66)
7 3 5 5
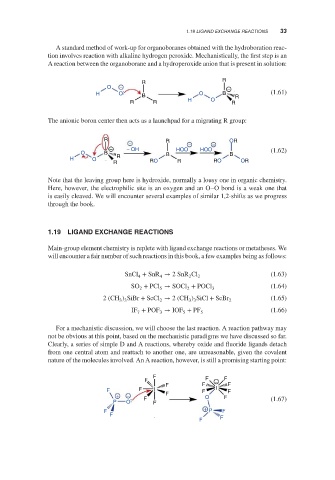
For a mechanistic discussion, we will choose the last reaction. A reaction pathway may
not be obvious at this point, based on the mechanistic paradigms we have discussed so far.
Clearly, a series of simple D and A reactions, whereby oxide and fluoride ligands detach
from one central atom and reattach to another one, are unreasonable, given the covalent
nature of the molecules involved. An A reaction, however, is still a promising starting point:
F
F F − F
F F F
F F I F I F
+ − F O F
P O F F (1.67)
+
F P F
F
F F