Page 57 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 57
1.21 PERICYCLIC REACTIONS 37
Although much less commonly used in chemical synthesis relative to polar reactions,
radical reactions nonetheless form a distinct “genre” of synthetic reactions. Creatively
orchestrated, they can lead to a variety of complex structures with a surprising degree of
efficiency.
1.21 PERICYCLIC REACTIONS
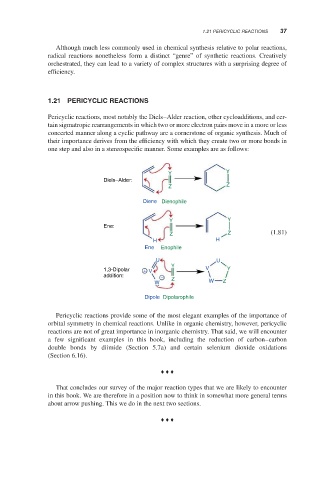
Pericyclic reactions, most notably the Diels–Alder reaction, other cycloadditions, and cer-
tain sigmatropic rearrangements in which two or more electron pairs move in a more or less
concerted manner along a cyclic pathway are a cornerstone of organic synthesis. Much of
their importance derives from the efficiency with which they create two or more bonds in
one step and also in a stereospecific manner. Some examples are as follows:
Y Y
Diels–Alder:
Z Z
Diene Dienophile
Y Y
Ene:
Z Z (1.81)
H H
Ene Enophile
U U
Y
1,3-Dipolar + V V Y
addition: − Z
W W Z
Dipole Dipolarophile
Pericyclic reactions provide some of the most elegant examples of the importance of
orbital symmetry in chemical reactions. Unlike in organic chemistry, however, pericyclic
reactions are not of great importance in inorganic chemistry. That said, we will encounter
a few significant examples in this book, including the reduction of carbon–carbon
double bonds by diimide (Section 5.7a) and certain selenium dioxide oxidations
(Section 6.16).
♦♦♦
That concludes our survey of the major reaction types that we are likely to encounter
in this book. We are therefore in a position now to think in somewhat more general terms
about arrow pushing. This we do in the next two sections.
♦♦♦