Page 60 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 60
A COLLECTION OF BASIC CONCEPTS
40
It’s time now to think about hypervalent compounds. You have encountered a few of them
already, as products of A reactions and as intermediates in S 2-Si mechanisms. But what is
N
special about such compounds? Is the term “hypervalent” synonymous with higher-valent?
(No.) To better understand these issues, we’ll take a step back in Section 1.24 and remind
ourselves what the term “valence” exactly means and how it differs from related concepts
such as coordination number (CN), FC, and oxidation state (OS). Confusion between these
terms and incorrect usage are widespread in both textbooks and the research literature.
From there we’ll proceed on to some related topics such as an elementary molecular orbital
description of hypervalent bonding (Section 1.25). We’ll conclude this chapter with a brief
discussion of the inert pair effect, an important aspect of the variable valence of the heaviest
(sixth-period) p-block elements.
♦♦♦
DEFINITIONS: VALENCE, OXIDATION STATE, FORMAL CHARGE,
1.24
AND COORDINATION NUMBER
Table 1.7 presents compact definitions for all four concepts.
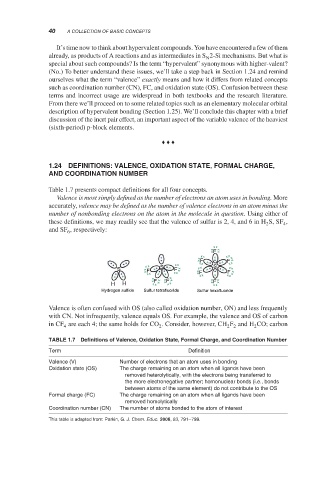
Valence is most simply defined as the number of electrons an atom uses in bonding. More
accurately, valence may be defined as the number of valence electrons in an atom minus the
number of nonbonding electrons on the atom in the molecule in question. Using either of
these definitions, we may readily see that the valence of sulfur is 2, 4, and 6 in H S, SF ,
2
4
and SF , respectively:
6
F xx x x
xx
x x x x x
F x x
x x
F x x
x x x x
x x S x x S
S x x F x x F x x x x F x x x x x
x x x x x x x x x xx x xx
F x
x
F x x xx F x x
x
x x
H H x x x x x x
x x
F x
Hydrogen sulfide Sulfur tetrafluoride Sulfur hexafluoride
Valence is often confused with OS (also called oxidation number, ON) and less frequently
with CN. Not infrequently, valence equals OS. For example, the valence and OS of carbon
in CF are each 4; the same holds for CO . Consider, however, CH F and H CO; carbon
4
2 2
2
2
TABLE 1.7 Definitions of Valence, Oxidation State, Formal Charge, and Coordination Number
Term Definition
Valence (V) Number of electrons that an atom uses in bonding
Oxidation state (OS) The charge remaining on an atom when all ligands have been
removed heterolytically, with the electrons being transferred to
the more electronegative partner; homonuclear bonds (i.e., bonds
between atoms of the same element) do not contribute to the OS
Formal charge (FC) The charge remaining on an atom when all ligands have been
removed homolytically
Coordination number (CN) The number of atoms bonded to the atom of interest
This table is adapted from: Parkin, G. J. Chem. Educ. 2006, 83, 791–799.