Page 64 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 64
A COLLECTION OF BASIC CONCEPTS
44
− − 2−
O O N
+ 2+ 3+ 2+
P − S S S
HO OH − − − F
HO O O O O F F
H 3 PO 4 SO 2 SO 3 SNF 3
− −
O O
− −
− O 2+ O 2+ O − − Cl 3+ Xe 4+ −
O O O − − O
I I O O O −
O
−
−
I 2 O 5 ClO 4 XeO 4
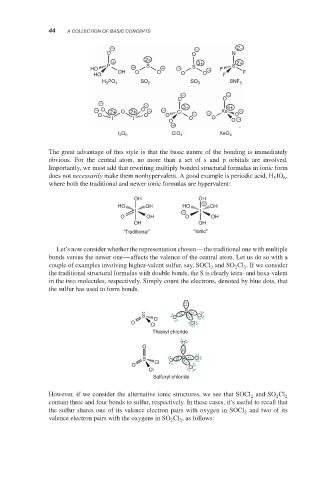
The great advantage of this style is that the basic nature of the bonding is immediately
obvious. For the central atom, no more than a set of s and p orbitals are involved.
Importantly, we must add that rewriting multiply bonded structural formulas in ionic form
does not necessarily make them nonhypervalent. A good example is periodic acid, H IO ,
6
5
where both the traditional and newer ionic formulas are hypervalent:
OH OH
+
HO OH HO OH
I − I
O OH O OH
OH OH
“Traditional” “Ionic”
Let’s now consider whether the representation chosen—the traditional one with multiple
bonds versus the newer one—affects the valence of the central atom. Let us do so with a
couple of examples involving higher-valent sulfur, say, SOCl and SO Cl . If we consider
2
2
2
the traditional structural formulas with double bonds, the S is clearly tetra- and hexa-valent
in the two molecules, respectively. Simply count the electrons, denoted by blue dots, that
the sulfur has used to form bonds.
S x x
S x x O xx x x Cl x
Cl xx x x x
O Cl x x xx x
Cl x
Thionyl chloride
O x x
x x
O
xx
x x
x x S
S O xx x Cl x x
x x
O Cl x x x Cl x x
Cl x x xx
Sulfuryl chloride
However, if we consider the alternative ionic structures, we see that SOCl and SO Cl
2 2 2
contain three and four bonds to sulfur, respectively. In these cases, it’s useful to recall that
the sulfur shares one of its valence electron pairs with oxygen in SOCl and two of its
2
valence electron pairs with the oxygens in SO Cl , as follows:
2 2