Page 62 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 62
A COLLECTION OF BASIC CONCEPTS
42
2− xx xx −
xx
xx F xx xx F xx xx F xx
x x x x x x x x xx x F x x x x x x x
xx
x F x x F
x x
xx
xx
x
xx F x x x x xx
x
F xx
Si x P P
F x x
x
x x x
x x xx x xx F x
F F
xx
xx
x x
x x
x
x
x F x x x x x x xx x xx xx
xx
F xx
x x
xx F
xx F
F xx xx xx
xx
xx xx xx
Hexafluorosilicate Phosphorus pentafluoride Hexafluorophosphate
2−
SiF 6 PF 5 PF 6
xx
xx F xx xx xx x x xx F xx
xx
xx F xx F x x x x x x xx
x
F xx x Br x x x x x x x x x x x F x x x I x F xx
xx
x
I x F x xx x x x x F xx
x x
x x
F x
x
x
x x x x x x x x x
x
xx
F xx xx F xx x x F x x F xx xx F xx
xx xx
xx
Bromine trifluoride Iodine pentafluoride Iodine heptafluoride
BrF 3
IF 5 IF 7
2−
xx F xx xx x x F x x x x x x F x x x x
x x
x x x x x xx F xx x x F x x
x x
x x F
x F xx x x x x
x x
x x
x
x F x x x x Xe x x x x Xe xx
xx F
xx x x x F x x F x x
xx
Xe x F xx Xe F x x x x x x x
xx F x x x x xx F xx x x x x x xx
x
xx x x x x x x xx x xx x x x x F
x F F xx x xx xx F x x x x x xF x x
xx
x
x x
x x x
Xenon difluoride Xenon tetrafluoride Xenon hexafluoride Octafluoroxenate(VI)
2−
XeF 2 XeF 4 XeF 6 XeF 8
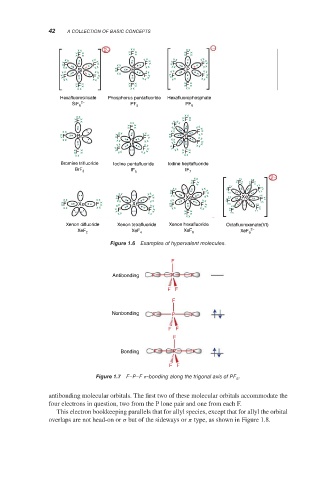
Examples of hypervalent molecules.
Figure 1.6
F
Antibonding F P F
F F
F
Nonbonding F P F
F F
F
Bonding F P F
F F
Figure 1.7 F–P–F -bonding along the trigonal axis of PF .
5
antibonding molecular orbitals. The first two of these molecular orbitals accommodate the
four electrons in question, two from the P lone pair and one from each F.
This electron bookkeeping parallels that for allyl species, except that for allyl the orbital
overlaps are not head-on or but of the sideways or type, as shown in Figure 1.8.