Page 66 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 66
A COLLECTION OF BASIC CONCEPTS
46
nonstandard valence states of p-block elements. The superscript in this notation equals
the valence of the atom in question, as defined in Table 1.7. Thus, tri- and penta-valent
3
5
phosphorus compounds are called - and -phosphoranes, respectively; tetra- and
4
6
hexa-valent sulfur compounds are called - and -sulfuranes, and so on. The system
is particularly useful for organoiodine compounds, where the iodine can exhibit a
variety of different valence states. Thus, the hypothetical parent compounds H I, H I,
3
5
5
3
7
and H I would be known as -, -, and -iodane, respectively. To take a couple
7
+
of slightly more complicated examples, a phosphonium ylide such as Ph P CH 2 − is
3
5
called -(methylene)triphenylphosphorane in this system; similarly, the sulfur ylide
+
4
(CH ) S CH 2 − is called -(methylene)dimethylsulfurane. We will use this nomenclature
3 2
from time to time as we progress through the p-block elements.
1.27 THE INERT PAIR EFFECT
The inert pair effect is another important concept to be aware of, especially in connection
with the heavier main-group elements. It refers to the increasing tendency of the outermost
s electrons to remain un-ionized or unshared for the heaviest p-block elements. The origin
of the effect is rather complex, involving differences in nuclear screening and relativistic
stabilization, as experienced by the outermost s versus p electrons, and is not greatly relevant
to our discussion. The chemical consequences of the inert pair effect, on the other hand, are
striking and particularly important for groups 13 (In, Tl), 14 (Sn, Pb), and 15 (Sb, Bi); the
following examples should illustrate the point.
While the chemistry of aluminum is largely that of the trivalent state, the monovalent
state becomes increasingly stable as one goes down group 13; for thallium, the monovalent
state is the common state. Trivalent Tl salts are reactive and used as oxidants in organic
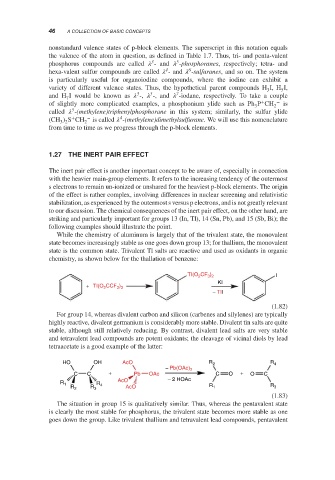
chemistry, as shown below for the thallation of benzene:
TI(O CF ) I
3 2
2
KI
+ TI(O CCF )
3 3
2
− TII
(1.82)
For group 14, whereas divalent carbon and silicon (carbenes and silylenes) are typically
highly reactive, divalent germanium is considerably more stable. Divalent tin salts are quite
stable, although still relatively reducing. By contrast, divalent lead salts are very stable
and tetravalent lead compounds are potent oxidants; the cleavage of vicinal diols by lead
tetraacetate is a good example of the latter:
HO OH AcO R 2 R 4
− Pb(OAc) 2
C C + Pb OAc C O + O C
AcO − 2 HOAc
R 1 R 4 R
R 2 R 3 AcO R 1 3
(1.83)
The situation in group 15 is qualitatively similar. Thus, whereas the pentavalent state
is clearly the most stable for phosphorus, the trivalent state becomes more stable as one
goes down the group. Like trivalent thallium and tetravalent lead compounds, pentavalent