Page 49 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 49
1.16 CARBENES 29
1.16 CARBENES
Divalent carbon species, or carbenes, are another classic group of organic intermediates.
Not only are they important in organic chemistry but they are also isoelectronic with other
divalent group 14 molecules (based on Ge, Sn, and Pb) and hence of considerable rele-
vance to our further discussions. In addition, nitrogen-stabilized, so-called N-heterocyclic
carbenes (NHCs) have gained enormous popularity as transition-metal ligands; many of the
resulting complexes mediate unique transformations that define a frontier area of contem-
porary chemistry.
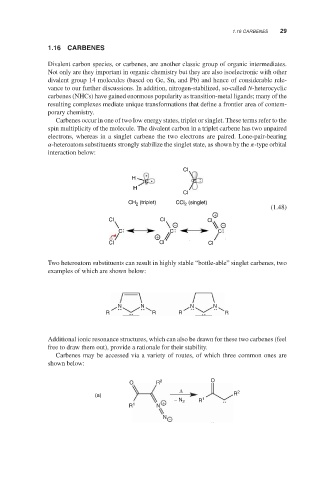
Carbenes occur in one of two low energy states, triplet or singlet. These terms refer to the
spin multiplicity of the molecule. The divalent carbon in a triplet carbene has two unpaired
electrons, whereas in a singlet carbene the two electrons are paired. Lone-pair-bearing
-heteroatom substituents strongly stabilize the singlet state, as shown by the -type orbital
interaction below:
Cl
H
C C
H
Cl
CH (triplet) CCl (singlet)
2
2
(1.48)
+
Cl Cl Cl
− −
C C C
+
Cl Cl Cl
Two heteroatom substituents can result in highly stable “bottle-able” singlet carbenes, two
examples of which are shown below:
N N N N
R R R R
Additional ionic resonance structures, which can also be drawn for these two carbenes (feel
free to draw them out), provide a rationale for their stability.
Carbenes may be accessed via a variety of routes, of which three common ones are
shown below:
O R 2 O
Δ 2
(a) R
+ − N 2 R 1
R 1 N
N −