Page 46 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 46
A COLLECTION OF BASIC CONCEPTS
26
18
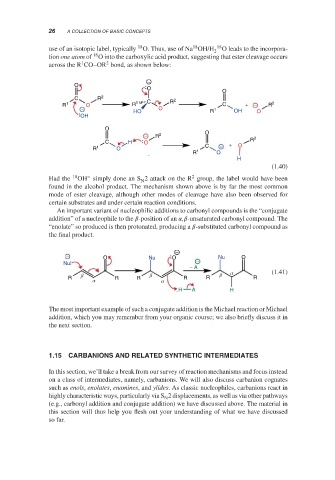
use of an isotopic label, typically 18 O. Thus, use of Na OH/H 18 O leads to the incorpora-
2
18
tion one atom of O into the carboxylic acid product, suggesting that ester cleavage occurs
2
1
across the R CO–OR bond, as shown below:
−
O
O
O
C R 2 2
R 1 O R 1 C R C + − R 2
− HO O R 1 OH O
OH
O
− R 2 O 2
C H O + R
R 1 O 1 C − O
R O
H
(1.40)
−
2
Had the 18 OH simply done an S 2 attack on the R group, the label would have been
N
found in the alcohol product. The mechanism shown above is by far the most common
mode of ester cleavage, although other modes of cleavage have also been observed for
certain substrates and under certain reaction conditions.
An important variant of nucleophilic additions to carbonyl compounds is the “conjugate
addition” of a nucleophile to the -position of an , -unsaturated carbonyl compound. The
“enolate” so produced is then protonated, producing a -substituted carbonyl compound as
the final product.
−
− O Nu O Nu O
Nu −
− A
β β β α (1.41)
R α R R α R R R
H A H
The most important example of such a conjugate addition is the Michael reaction or Michael
addition, which you may remember from your organic course; we also briefly discuss it in
the next section.
1.15 CARBANIONS AND RELATED SYNTHETIC INTERMEDIATES
In this section, we’ll take a break from our survey of reaction mechanisms and focus instead
on a class of intermediates, namely, carbanions. We will also discuss carbanion cognates
such as enols, enolates, enamines, and ylides. As classic nucleophiles, carbanions react in
highly characteristic ways, particularly via S 2 displacements, as well as via other pathways
N
(e.g., carbonyl addition and conjugate addition) we have discussed above. The material in
this section will thus help you flesh out your understanding of what we have discussed
so far.