Page 41 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 41
1.11 TWO-STEP IONIC MECHANISMS: THE S 1 AND E1 PATHWAYS
N 21
H 3 C
+ H 3 C
C CH 3
H 3 C
S N 1
H C C OAc
H 3 C − H − OAc H
+ OAc H C
C CH 3 H
H
H H 3 C
C (1.27)
H − +
H C CH 3 H 3 C
OAc
E1 H − HOAc
C C CH 3
H
H H C
H
−
OAc
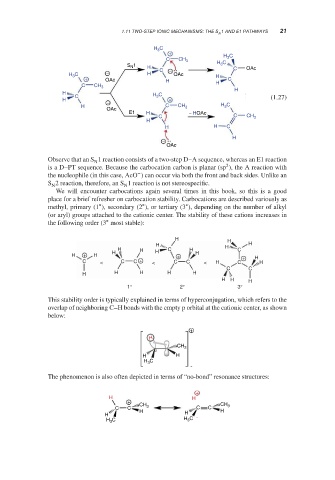
Observe that an S 1 reaction consists of a two-step D–A sequence, whereas an E1 reaction
N
2
is a D–PT sequence. Because the carbocation carbon is planar (sp ), the A reaction with
−
the nucleophile (in this case, AcO ) can occur via both the front and back sides. Unlike an
S 2 reaction, therefore, an S 1 reaction is not stereospecific.
N
N
We will encounter carbocations again several times in this book, so this is a good
place for a brief refresher on carbocation stability. Carbocations are described variously as
∘
∘
∘
methyl, primary (1 ), secondary (2 ), or tertiary (3 ), depending on the number of alkyl
(or aryl) groups attached to the cationic center. The stability of these cations increases in
∘
the following order (3 most stable):
H H
H H
H H C H H C
H H H
H + H + + H
C < C C + < C C < H C H
C C
H H H H H
H H H
1° 2° 3°
This stability order is typically explained in terms of hyperconjugation, which refers to the
overlap of neighboring C–H bonds with the empty p orbital at the cationic center, as shown
below:
+
H
CH 3
H H
H 3 C
The phenomenon is also often depicted in terms of “no-bond” resonance structures:
+
H H
+
CH 3 CH 3
C C C C
H H
H H
H 3 C H 3 C