Page 39 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 39
1.10 TWO-STEP IONIC MECHANISMS: THE S 2-Si PATHWAY
N 19
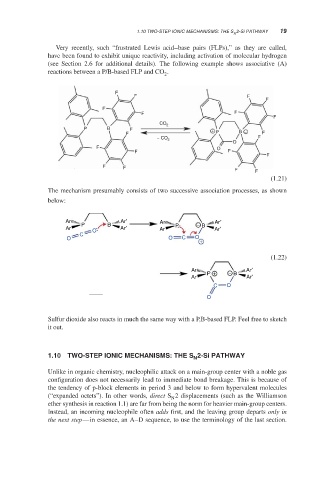
Very recently, such “frustrated Lewis acid–base pairs (FLPs),” as they are called,
have been found to exhibit unique reactivity, including activation of molecular hydrogen
(see Section 2.6 for additional details). The following example shows associative (A)
reactions between a P/B-based FLP and CO .
2
F
F F
F
F F
F F
F
CO
2
P B F +
F P B − F
F
− CO 2
O
F O
F F
F
F F
F F
(1.21)
The mechanism presumably consists of two successive association processes, as shown
below:
Ar P B Ar′ Ar − Ar′
Ar O Ar′ Ar P B Ar′
C
O O C O
+
(1.22)
Ar P + − B Ar′
Ar Ar′
C O
O
Sulfur dioxide also reacts in much the same way with a P,B-based FLP. Feel free to sketch
it out.
1.10 TWO-STEP IONIC MECHANISMS: THE S 2-Si PATHWAY
N
Unlike in organic chemistry, nucleophilic attack on a main-group center with a noble gas
configuration does not necessarily lead to immediate bond breakage. This is because of
the tendency of p-block elements in period 3 and below to form hypervalent molecules
(“expanded octets”). In other words, direct S 2 displacements (such as the Williamson
N
ether synthesis in reaction 1.1) are far from being the norm for heavier main-group centers.
Instead, an incoming nucleophile often adds first, and the leaving group departs only in
the next step—in essence, an A–D sequence, to use the terminology of the last section.