Page 36 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 36
A COLLECTION OF BASIC CONCEPTS
16
+ R 1 H
O
R 1 H O
(a) H H O + +
O H H
R 2
(1.11)
R 2 + +
O O
(b) H R C N H +
H H H
N H
C
R
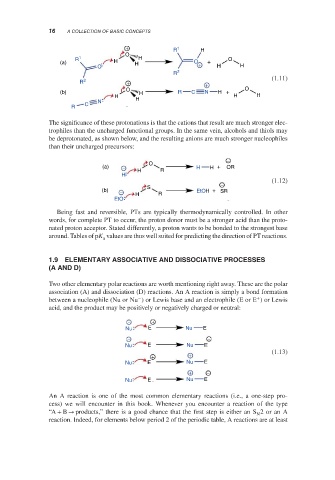
The significance of these protonations is that the cations that result are much stronger elec-
trophiles than the uncharged functional groups. In the same vein, alcohols and thiols may
be deprotonated, as shown below, and the resulting anions are much stronger nucleophiles
than their uncharged precursors:
−
O
(a) − H H + OR
H R
H
(1.12)
−
S
(b) − H R EtOH + SR
EtO
Being fast and reversible, PTs are typically thermodynamically controlled. In other
words, for complete PT to occur, the proton donor must be a stronger acid than the proto-
nated proton acceptor. Stated differently, a proton wants to be bonded to the strongest base
around. Tables of pK values are thus well suited for predicting the direction of PT reactions.
a
1.9 ELEMENTARY ASSOCIATIVE AND DISSOCIATIVE PROCESSES
(A AND D)
Two other elementary polar reactions are worth mentioning right away. These are the polar
association (A) and dissociation (D) reactions. An A reaction is simply a bond formation
− +
between a nucleophile (Nu or Nu ) or Lewis base and an electrophile (E or E )orLewis
acid, and the product may be positively or negatively charged or neutral:
− +
Nu E Nu E
− −
Nu E Nu E
(1.13)
+ +
Nu E Nu E
+ −
Nu E Nu E
An A reaction is one of the most common elementary reactions (i.e., a one-step pro-
cess) we will encounter in this book. Whenever you encounter a reaction of the type
“A + B → products,” there is a good chance that the first step is either an S 2oranA
N
reaction. Indeed, for elements below period 2 of the periodic table, A reactions are at least