Page 42 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 42
A COLLECTION OF BASIC CONCEPTS
22
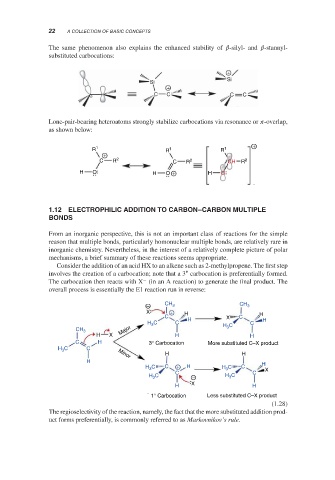
The same phenomenon also explains the enhanced stability of -silyl- and -stannyl-
substituted carbocations:
+
Si
Si
+
C C C C
Lone-pair-bearing heteroatoms strongly stabilize carbocations via resonance or -overlap,
as shown below:
+
R 1 R 1 R 1
+
C R 2 C R 2 CH R 2
H O H O + H O
1.12 ELECTROPHILIC ADDITION TO CARBON–CARBON MULTIPLE
BONDS
From an inorganic perspective, this is not an important class of reactions for the simple
reason that multiple bonds, particularly homonuclear multiple bonds, are relatively rare in
inorganic chemistry. Nevertheless, in the interest of a relatively complete picture of polar
mechanisms, a brief summary of these reactions seems appropriate.
Consider the addition of an acid HX to an alkene such as 2-methylpropene. The first step
∘
involves the creation of a carbocation; note that a 3 carbocation is preferentially formed.
−
The carbocation then reacts with X (in an A reaction) to generate the final product. The
overall process is essentially the E1 reaction run in reverse:
− CH 3 CH 3
X + H
C H X C H H
H 3 C C H C C
3
CH 3 Major
H X H H
C H 3° Carbocation More substituted C–X product
H C C
3
H H
Minor
H H
C C + H C C
H 3 H 3 X
C C
H C − H C
3
3
H X H
1° Carbocation Less substituted C–X product
(1.28)
The regioselectivity of the reaction, namely, the fact that the more substituted addition prod-
uct forms preferentially, is commonly referred to as Markovnikov’s rule.