Page 45 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 45
1.14 NUCLEOPHILIC ADDITION TO CARBON–HETEROATOM MULTIPLE BONDS 25
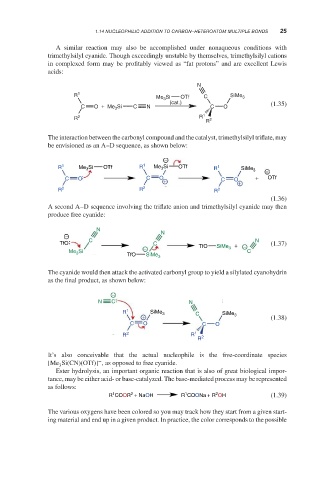
A similar reaction may also be accomplished under nonaqueous conditions with
trimethylsilyl cyanide. Though exceedingly unstable by themselves, trimethylsilyl cations
in complexed form may be profitably viewed as “fat protons” and are excellent Lewis
acids:
N
R 1 Me Si OTf C SiMe 3
3
(cat.) (1.35)
C O + Me Si C N C O
3
R 2 R 1 2
R
The interaction between the carbonyl compound and the catalyst, trimethylsilyl triflate, may
be envisioned as an A–D sequence, as shown below:
−
R 1 Me 3 Si OTf R 1 Me 3 Si OTf R 1
−
SiMe 3
C O C O + C O + + OTf
R 2 R 2 R 2
(1.36)
A second A–D sequence involving the triflate anion and trimethylsilyl cyanide may then
produce free cyanide:
N
− N
C N
TfO C + (1.37)
− TfO SiMe 3 −
Me 3 Si C
TfO SiMe 3
The cyanide would then attack the activated carbonyl group to yield a silylated cyanohydrin
as the final product, as shown below:
−
N C N
R 1 SiMe 3 C SiMe 3
+ (1.38)
C O C O
R 2 R 1 2
R
It’s also conceivable that the actual nucleophile is the five-coordinate species
−
[Me Si(CN)(OTf)] , as opposed to free cyanide.
3
Ester hydrolysis, an important organic reaction that is also of great biological impor-
tance, may be either acid- or base-catalyzed. The base-mediated process may be represented
as follows:
2
2
1
1
R COOR + NaOH R COONa + R OH (1.39)
The various oxygens have been colored so you may track how they start from a given start-
ing material and end up in a given product. In practice, the color corresponds to the possible