Page 27 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 27
1.2 WHAT MAKES FOR A GOOD NUCLEOPHILE? 7
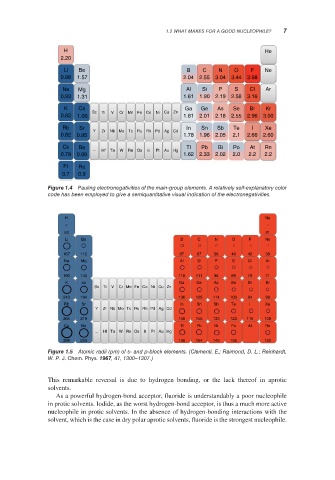
H He
2.20
Li Be B C N O F Ne
0.98 1.57 2.04 2.55 3.04 3.44 3.98
Na Mg Al Si P S Cl Ar
0.93 1.31 1.61 1.90 2.19 2.58 3.16
K Ca Ga Ge As Se Br Kr
Sc Ti V Cr Mn Fe Co Ni Cu Zn
0.82 1.00 1.81 2.01 2.18 2.55 2.96 3.00
Rb Sr In Sn Sb Te I Xe
Y Zr Nb Mo Tc Ru Rh Pd Ag Cd
0.82 0.95 1.78 1.96 2.05 2.1 2.66 2.60
Cs Ba Tl Pb Bi Po At Rn
– Hf Ta W Re Os Ir Pt Au Hg
0.79 0.89 1.62 2.33 2.02 2.0 2.2 2.2
Fr Ra
0.7 0.9
Figure 1.4 Pauling electronegativities of the main-group elements. A relatively self-explanatory color
code has been employed to give a semiquantitative visual indication of the electronegativities.
H Ne
53 31
Li Be B C N O F Ne
167 112 87 67 56 48 42 38
Na Mg Al Si P S Cl Ar
190 145 118 111 98 88 79 71
K ca Ga Ge As Se Br Kr
Sc Ti V Cr Mn Fe Co Ni Cu Zn
243 194 136 125 114 103 94 88
Rb Sr In Sn Sb Te I Xe
Y Zr Nb Mo Tc Ru Rh Pd Ag Cd
265 219 156 145 133 123 115 108
Cs Ba Tl Pb Bi Po At Rn
– Hf Ta W Re Os Ir Pt Au Hg
298 253 156 154 143 135 120
Figure 1.5 Atomic radii (pm) of s- and p-block elements. (Clementi, E.; Raimond, D. L.; Reinhardt,
W. P. J. Chem. Phys. 1967, 47, 1300–1307.)
This remarkable reversal is due to hydrogen bonding, or the lack thereof in aprotic
solvents.
As a powerful hydrogen-bond acceptor, fluoride is understandably a poor nucleophile
in protic solvents. Iodide, as the worst hydrogen-bond acceptor, is thus a much more active
nucleophile in protic solvents. In the absence of hydrogen-bonding interactions with the
solvent, which is the case in dry polar aprotic solvents, fluoride is the strongest nucleophile.