Page 166 - Artificial Intelligence for Computational Modeling of the Heart
P. 166
138 Chapter 4 Data-driven reduction of cardiac models
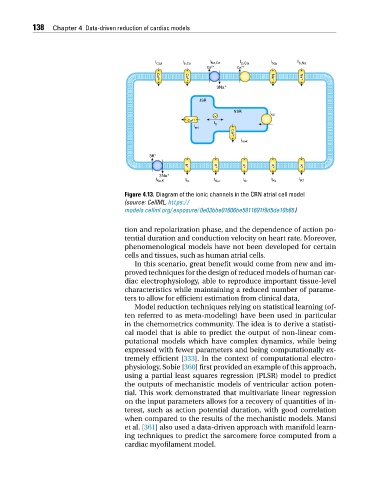
Figure 4.13. Diagram of the ionic channels in the CRN atrial cell model
(source: CellML, https:/ /
models.cellml.org/ exposure/ 0e03bbe01606be5811691f9d5de10b65).
tion and repolarization phase, and the dependence of action po-
tential duration and conduction velocity on heart rate. Moreover,
phenomenological models have not been developed for certain
cells and tissues, such as human atrial cells.
In this scenario, great benefit would come from new and im-
proved techniques for the design of reduced models of human car-
diac electrophysiology, able to reproduce important tissue-level
characteristics while maintaining a reduced number of parame-
ters to allow for efficient estimation from clinical data.
Model reduction techniques relying on statistical learning (of-
ten referred to as meta-modeling) have been used in particular
in the chemometrics community. The idea is to derive a statisti-
cal model that is able to predict the output of non-linear com-
putational models which have complex dynamics, while being
expressed with fewer parameters and being computationally ex-
tremely efficient [333]. In the context of computational electro-
physiology, Sobie [360] first provided an example of this approach,
using a partial least squares regression (PLSR) model to predict
the outputs of mechanistic models of ventricular action poten-
tial. This work demonstrated that multivariate linear regression
on the input parameters allows for a recovery of quantities of in-
terest, such as action potential duration, with good correlation
when compared to the results of the mechanistic models. Mansi
et al. [361] also used a data-driven approach with manifold learn-
ing techniques to predict the sarcomere force computed from a
cardiac myofilament model.