Page 108 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 108
92 Assurance of sterility for sensitive combination products and materials
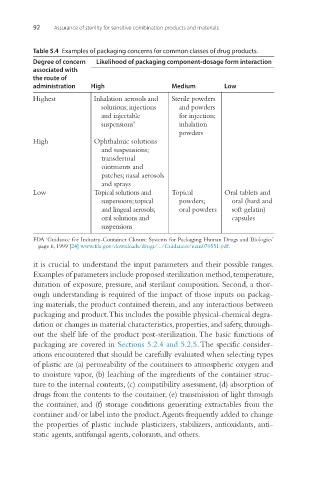
Table 5.4 Examples of packaging concerns for common classes of drug products.
Degree of concern Likelihood of packaging component-dosage form interaction
associated with
the route of
administration High Medium Low
Highest Inhalation aerosols and Sterile powders
solutions; injections and powders
and injectable for injection;
suspensions a inhalation
powders
High Ophthalmic solutions
and suspensions;
transdermal
ointments and
patches; nasal aerosols
and sprays
Low Topical solutions and Topical Oral tablets and
suspensions; topical powders; oral (hard and
and lingual aerosols; oral powders soft gelatin)
oral solutions and capsules
suspensions
FDA ‘Guidance for Industry-Container Closure Systems for Packaging Human Drugs and Biologics’
page 6, 1999 [24] www.fda.gov/downloads/drugs/.../Guidances/ucm070551.pdf.
it is crucial to understand the input parameters and their possible ranges.
Examples of parameters include proposed sterilization method, temperature,
duration of exposure, pressure, and sterilant composition. Second, a thor-
ough understanding is required of the impact of those inputs on packag-
ing materials, the product contained therein, and any interactions between
packaging and product. This includes the possible physical-chemical degra-
dation or changes in material characteristics, properties, and safety, through-
out the shelf life of the product post-sterilization. The basic functions of
packaging are covered in Sections 5.2.4 and 5.2.5. The specific consider-
ations encountered that should be carefully evaluated when selecting types
of plastic are (a) permeability of the containers to atmospheric oxygen and
to moisture vapor, (b) leaching of the ingredients of the container struc-
ture to the internal contents, (c) compatibility assessment, (d) absorption of
drugs from the contents to the container, (e) transmission of light through
the container, and (f) storage conditions generating extractables from the
container and/or label into the product. Agents frequently added to change
the properties of plastic include plasticizers, stabilizers, antioxidants, anti-
static agents, antifungal agents, colorants, and others.