Page 16 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 16
6
Assurance of sterility for sensitive combination products and materials
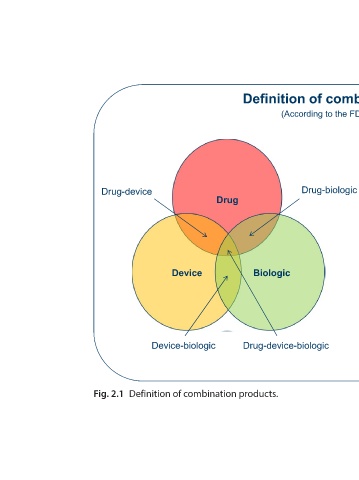
Definition of combination products
(According to the FDA: 21 CFR 3.2(e)) A product comprised of two or more regulated components, 1. i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are physically, chemically, or otherwise combined or mixed and produced as a single entity; Two or more separate products packaged togeth
Biologic Drug-device-biologic
Drug
Device Device-biologic Definition of combination products.
Drug-device Fig. 2.1