Page 18 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 18
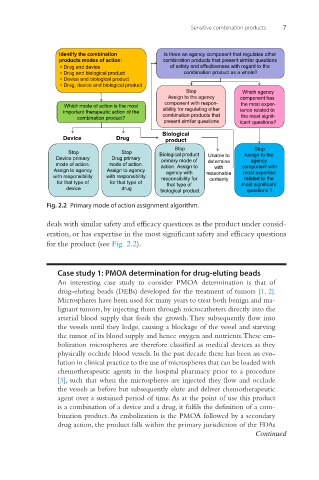
Sensitive combination products 7
Identify the combination Is there an agency component that regulates other
products modes of action: combination products that present similar questions
•Drug and device of safety and effectiveness with regard to the
• Drug and biological product combination product as a whole?
• Device and biological product
•Drug, device and biological product
Stop Which agency
Assign to the agency component has
component with respon- the most exper-
Which mode of action is the most
important therapeutic action of the sibility for regulating other ience related to
combination product? combination products that the most signif-
present similar questions icant questions?
Biological
Device Drug product
Stop Stop
Stop Stop Biological product Assign to the
Device primary Drug primary primary mode of Unable to agency
mode of action. mode of action. action. Assign to determine component with
Assign to agency Assign to agency agency with with most expertise
with responsibility with responsibility responsibility for reasonable related to the
certainty
for that type of for that type of that type of most significant
device drug biological product questions ?
Fig. 2.2 Primary mode of action assignment algorithm.
deals with similar safety and efficacy questions as the product under consid-
eration, or has expertise in the most significant safety and efficacy questions
for the product (see Fig. 2.2).
Case study 1: PMOA determination for drug-eluting beads
An interesting case study to consider PMOA determination is that of
drug-eluting beads (DEBs) developed for the treatment of tumors [1, 2].
Microspheres have been used for many years to treat both benign and ma-
lignant tumors, by injecting them through microcatheters directly into the
arterial blood supply that feeds the growth. They subsequently flow into
the vessels until they lodge, causing a blockage of the vessel and starving
the tumor of its blood supply and hence oxygen and nutrients. These em-
bolization microspheres are therefore classified as medical devices as they
physically occlude blood vessels. In the past decade there has been an evo-
lution in clinical practice to the use of microspheres that can be loaded with
chemotherapeutic agents in the hospital pharmacy prior to a procedure
[3], such that when the microspheres are injected they flow and occlude
the vessels as before but subsequently elute and deliver chemotherapeutic
agent over a sustained period of time. As at the point of use this product
is a combination of a device and a drug, it fulfils the definition of a com-
bination product. As embolization is the PMOA followed by a secondary
drug action, the product falls within the primary jurisdiction of the FDAs
Continued