Page 22 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 22
Sensitive combination products 11
The simplest form of this type of combination product would take an
existing, approved drug and device and combine them. Ideally in this sce-
nario, the drug should also be approved for the indication in which is it be-
ing used, by the same route of administration and with the same mechanism
of action. There are normally, however, a range of new questions that arise as
a consequence of using a device to mediate local delivery of a therapeutic
that must be addressed in order to demonstrate continued safety and effi-
cacy of the combined product.
Case study 2: Sterilization of DES
The drug may be distributed throughout the device itself (as for the DEB or
an antibiotic-containing cement) or be contained in matrix such as a bio-
medical polymer coating, usually at the interface where the device would
contact the body. The DES commonly employ this approach which allows
for a consistent dose of drug to be applied along the length of the stent and
concentrated on the outer surface where the device expands into contact
with the vessel wall. There are a wide variety of biomedical polymers in use
in these types of applications, some of which are biostable and inert, others
are designed to bioresorb within the body over defined time period. In the
case of biodegradable polymers, the matrices themselves may be sensitive to
the processes of sterilization and premature degradation could be initiated
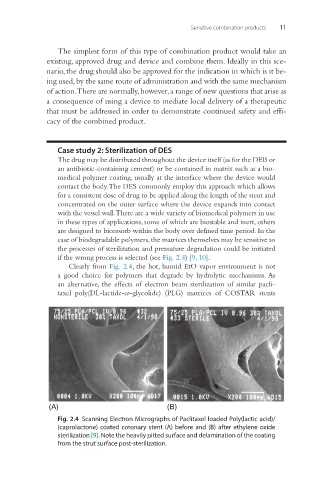
if the wrong process is selected (see Fig. 2.4) [9, 10].
Clearly from Fig. 2.4, the hot, humid EtO vapor environment is not
a good choice for polymers that degrade by hydrolytic mechanisms. As
an alternative, the effects of electron beam sterilization of similar pacli-
taxel poly(DL-lactide-co-glycolide) (PLG) matrices of COSTAR stents
(A) (B)
Fig. 2.4 Scanning Electron Micrographs of Paclitaxel loaded Poly(lactic acid)/
(caprolactone) coated coronary stent (A) before and (B) after ethylene oxide
sterilization [9]. Note the heavily pitted surface and delamination of the coating
from the strut surface post-sterilization.