Page 20 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 20
Sensitive combination products 9
Sterilisation
processes
Terminal sterilisation methods
Chemical Physical Physical
agents agents processes
Kills
Microbes
Gases: Liquids: Aseptic techniques
Ethylene oxide Peracetic acid filtration
Formaldehyde Gluteraldehyde
Nitrogen dioxide Chlorine Heat: Ionising
Steam (wet) radiation:
Hot air (dry) Gamma rays
Electron beam Excludes
X-rays microbes
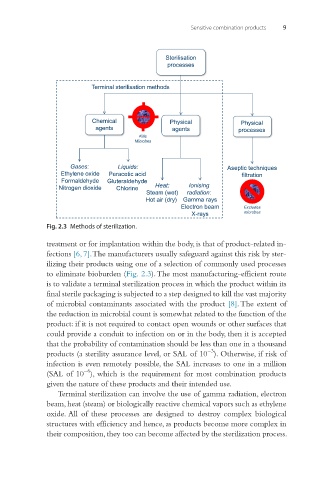
Fig. 2.3 Methods of sterilization.
treatment or for implantation within the body, is that of product-related in-
fections [6, 7]. The manufacturers usually safeguard against this risk by ster-
ilizing their products using one of a selection of commonly used processes
to eliminate bioburden (Fig. 2.3). The most manufacturing-efficient route
is to validate a terminal sterilization process in which the product within its
final sterile packaging is subjected to a step designed to kill the vast majority
of microbial contaminants associated with the product [8]. The extent of
the reduction in microbial count is somewhat related to the function of the
product: if it is not required to contact open wounds or other surfaces that
could provide a conduit to infection on or in the body, then it is accepted
that the probability of contamination should be less than one in a thousand
−3
products (a sterility assurance level, or SAL of 10 ). Otherwise, if risk of
infection is even remotely possible, the SAL increases to one in a million
−6
(SAL of 10 ), which is the requirement for most combination products
given the nature of these products and their intended use.
Terminal sterilization can involve the use of gamma radiation, electron
beam, heat (steam) or biologically reactive chemical vapors such as ethylene
oxide. All of these processes are designed to destroy complex biological
structures with efficiency and hence, as products become more complex in
their composition, they too can become affected by the sterilization process.