Page 26 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 26
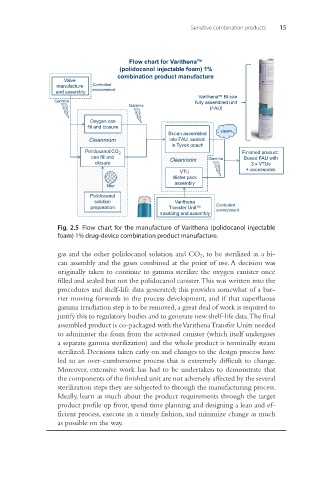
Sensitive combination products 15
Flow chart for Varithena™
(polidocanol injectable foam) 1%
combination product manufacture
Valve
manufacture Controlled
and assembly environment
Varithena™ Bi-can
Gamma fully assembled unit
Gamma
(FAU)
Oxygen can
fill and closure
Bi-can assembled steam
Cleanroom into FAU, sealed
in Tyvek pouch
Polidocanol:CO 2 Finished product:
can fill and Cleanroom Gamma Boxed FAU with
closure 3 x VTUs
VTU + accessories
blister pack
assembly
filter
Polidocanol
solution Varithena
preparation Transfer Unit™ Controlled
environment
moulding and assembly
Fig. 2.5 Flow chart for the manufacture of Varithena (polidocanol injectable
foam) 1% drug-device combination product manufacture.
gas and the other polidocanol solution and CO 2 , to be sterilized as a bi-
can assembly and the gases combined at the point of use. A decision was
originally taken to continue to gamma sterilize the oxygen canister once
filled and sealed but not the polidocanol canister. This was written into the
procedures and shelf-life data generated; this provides somewhat of a bar-
rier moving forwards in the process development, and if that superfluous
gamma irradiation step is to be removed, a great deal of work is required to
justify this to regulatory bodies and to generate new shelf-life data. The final
assembled product is co-packaged with the Varithena Transfer Units needed
to administer the foam from the activated canister (which itself undergoes
a separate gamma sterilization) and the whole product is terminally steam
sterilized. Decisions taken early on and changes to the design process have
led to an over-cumbersome process that is extremely difficult to change.
Moreover, extensive work has had to be undertaken to demonstrate that
the components of the finished unit are not adversely affected by the several
sterilization steps they are subjected to through the manufacturing process.
Ideally, learn as much about the product requirements through the target
product profile up front, spend time planning and designing a lean and ef-
ficient process, execute in a timely fashion, and minimize change as much
as possible on the way.