Page 31 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 31
Sensitive combination products 19
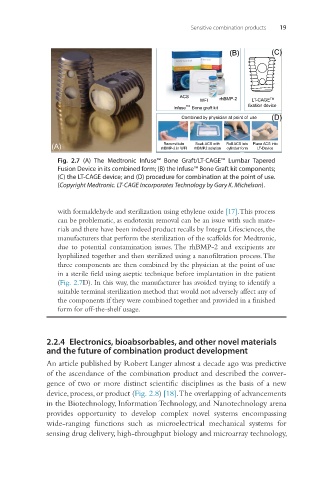
(B) (C)
ACS rhBMP-2
WFI LT-CAGE TM
Infuse TM Bone graft kit fixation device
Combined by physician at point of use (D)
Reconstitute
Soak ACS with
(A) rhBMP-2 in WFI rhBMP2 solution Roll ACS into Place ACS into
cylinder form
LT-Device
Fig. 2.7 (A) The Medtronic Infuse™ Bone Graft/LT-CAGE™ Lumbar Tapered
Fusion Device in its combined form; (B) the Infuse™ Bone Graft kit components;
(C) the LT-CAGE device; and (D) procedure for combination at the point of use.
(Copyright Medtronic. LT-CAGE Incorporates Technology by Gary K. Michelson).
with formaldehyde and sterilization using ethylene oxide [17]. This process
can be problematic, as endotoxin removal can be an issue with such mate-
rials and there have been indeed product recalls by Integra Lifesciences, the
manufacturers that perform the sterilization of the scaffolds for Medtronic,
due to potential contamination issues. The rhBMP-2 and excipients are
lyophilized together and then sterilized using a nanofiltration process. The
three components are then combined by the physician at the point of use
in a sterile field using aseptic technique before implantation in the patient
(Fig. 2.7D). In this way, the manufacturer has avoided trying to identify a
suitable terminal sterilization method that would not adversely affect any of
the components if they were combined together and provided in a finished
form for off-the-shelf usage.
2.2.4 Electronics, bioabsorbables, and other novel materials
and the future of combination product development
An article published by Robert Langer almost a decade ago was predictive
of the ascendance of the combination product and described the conver-
gence of two or more distinct scientific disciplines as the basis of a new
device, process, or product (Fig. 2.8) [18]. The overlapping of advancements
in the Biotechnology, Information Technology, and Nanotechnology arena
provides opportunity to develop complex novel systems encompassing
wide-ranging functions such as microelectrical mechanical systems for
sensing drug delivery, high-throughput biology and microarray technology,