Page 200 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 200
180 Assurance of sterility for sensitive combination products and materials
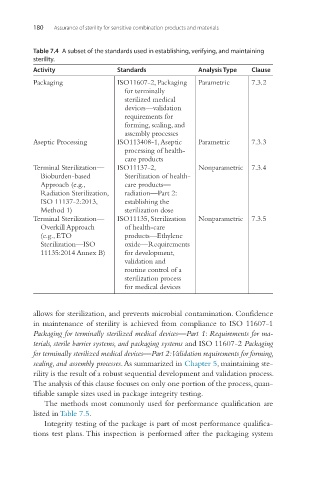
Table 7.4 A subset of the standards used in establishing, verifying, and maintaining
sterility.
Activity Standards Analysis Type Clause
Packaging ISO11607-2, Packaging Parametric 7.3.2
for terminally
sterilized medical
devices—validation
requirements for
forming, sealing, and
assembly processes
Aseptic Processing ISO113408-1, Aseptic Parametric 7.3.3
processing of health-
care products
Terminal Sterilization— ISO11137-2, Nonparametric 7.3.4
Bioburden-based Sterilization of health-
Approach (e.g., care products—
Radiation Sterilization, radiation—Part 2:
ISO 11137-2:2013, establishing the
Method 1) sterilization dose
Terminal Sterilization— ISO11135, Sterilization Nonparametric 7.3.5
Overkill Approach of health-care
(e.g., ETO products—Ethylene
Sterilization—ISO oxide—Requirements
11135:2014 Annex B) for development,
validation and
routine control of a
sterilization process
for medical devices
allows for sterilization, and prevents microbial contamination. Confidence
in maintenance of sterility is achieved from compliance to ISO 11607-1
Packaging for terminally sterilized medical devices—Part 1: Requirements for ma-
terials, sterile barrier systems, and packaging systems and ISO 11607-2 Packaging
for terminally sterilized medical devices—Part 2: Validation requirements for forming,
sealing, and assembly processes. As summarized in Chapter 5, maintaining ste-
rility is the result of a robust sequential development and validation process.
The analysis of this clause focuses on only one portion of the process, quan-
tifiable sample sizes used in package integrity testing.
The methods most commonly used for performance qualification are
listed in Table 7.5.
Integrity testing of the package is part of most performance qualifica-
tions test plans. This inspection is performed after the packaging system