Page 205 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 205
184 Assurance of sterility for sensitive combination products and materials
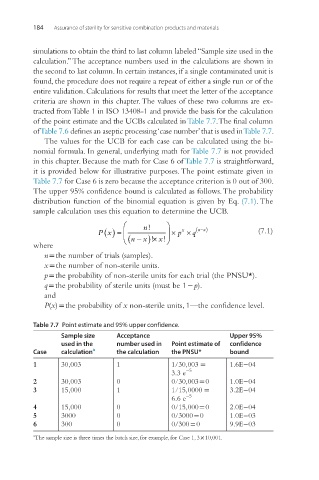
simulations to obtain the third to last column labeled “Sample size used in the
calculation.” The acceptance numbers used in the calculations are shown in
the second to last column. In certain instances, if a single contaminated unit is
found, the procedure does not require a repeat of either a single run or of the
entire validation. Calculations for results that meet the letter of the acceptance
criteria are shown in this chapter. The values of these two columns are ex-
tracted from Table 1 in ISO 13408-1 and provide the basis for the calculation
of the point estimate and the UCBs calculated in Table 7.7. The final column
of Table 7.6 defines an aseptic processing ‘case number’ that is used in Table 7.7.
The values for the UCB for each case can be calculated using the bi-
nomial formula. In general, underlying math for Table 7.7 is not provided
in this chapter. Because the math for Case 6 of Table 7.7 is straightforward,
it is provided below for illustrative purposes. The point estimate given in
Table 7.7 for Case 6 is zero because the acceptance criterion is 0 out of 300.
The upper 95% confidence bound is calculated as follows. The probability
distribution function of the binomial equation is given by Eq. (7.1). The
sample calculation uses this equation to determine the UCB.
n!
−
x
P x () = × p × q ( nx) (7.1)
( n − x) × ! x!
where
n = the number of trials (samples).
x = the number of non-sterile units.
p = the probability of non-sterile units for each trial (the PNSU*).
q = the probability of sterile units (must be 1 − p).
and
P(x) = the probability of x non-sterile units, 1—the confidence level.
Table 7.7 Point estimate and 95% upper confidence.
Sample size Acceptance Upper 95%
used in the number used in Point estimate of confidence
Case calculation a the calculation the PNSU* bound
1 30,003 1 1/30,003 = 1.6E−04
3.3 e −5
2 30,003 0 0/30,003 = 0 1.0E−04
3 15,000 1 1/15,0000 = 3.2E−04
6.6 e −5
4 15,000 0 0/15,000 = 0 2.0E−04
5 3000 0 0/3000 = 0 1.0E−03
6 300 0 0/300 = 0 9.9E−03
a The sample size is three times the batch size, for example, for Case 1, 3 × 10,001.