Page 57 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 57
Aseptic processing 45
Define Design Identify Control Commercial
product process variables strategy Qualify process production
What Prove the
What is How is Mitigate Run the
critical: achieved: could go the risk: process is process
wrong: capable
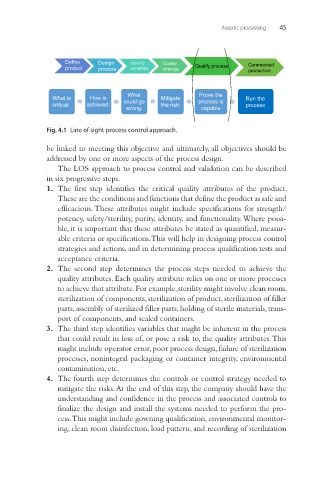
Fig. 4.1 Line of sight process control approach.
be linked to meeting this objective and ultimately, all objectives should be
addressed by one or more aspects of the process design.
The LOS approach to process control and validation can be described
in six progressive steps.
1. The first step identifies the critical quality attributes of the product.
These are the conditions and functions that define the product as safe and
efficacious. These attributes might include specifications for strength/
potency, safety/sterility, purity, identity, and functionality. Where possi-
ble, it is important that these attributes be stated as quantified, measur-
able criteria or specifications. This will help in designing process control
strategies and actions, and in determining process qualification tests and
acceptance criteria.
2. The second step determines the process steps needed to achieve the
quality attributes. Each quality attribute relies on one or more processes
to achieve that attribute. For example, sterility might involve clean room,
sterilization of components, sterilization of product, sterilization of filler
parts, assembly of sterilized filler parts, holding of sterile materials, trans-
port of components, and sealed containers.
3. The third step identifies variables that might be inherent in the process
that could result in loss of, or pose a risk to, the quality attributes. This
might include operator error, poor process design, failure of sterilization
processes, nonintegral packaging or container integrity, environmental
contamination, etc.
4. The fourth step determines the controls or control strategy needed to
mitigate the risks. At the end of this step, the company should have the
understanding and confidence in the process and associated controls to
finalize the design and install the systems needed to perform the pro-
cess. This might include gowning qualification, environmental monitor-
ing, clean room disinfection, load pattern, and recording of sterilization