Page 61 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 61
48 Assurance of sterility for sensitive combination products and materials
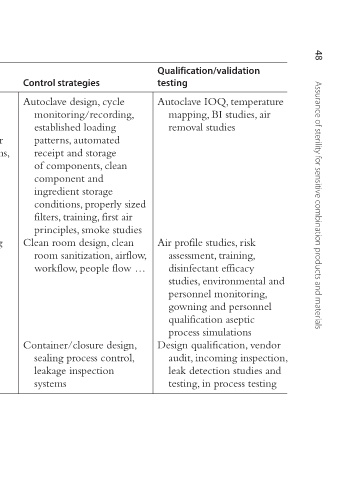
Qualification/validation testing Autoclave IOQ, temperature mapping, BI studies, air removal studies Air profile studies, risk assessment, training, disinfectant efficacy studies, environmental and personnel monitoring, gowning and personnel qualification aseptic process simulations Design qualification, ven
Control strategies Autoclave design, cycle monitoring/recording, established loading patterns, automated conditions, incorrect or receipt and storage random loading patterns, of components, clean component and ingredient storage conditions, properly sized filters, training, first air principles, smoke stu
Example of line of sight process evaluation. Process variables Process steps Inadequate temperature Sterilization and exposure time, air in system, steam excessive bioburden, incorrect components Poor aseptic and gowning Aseptic technique processing Leakage Container closure integrity
Table 4.1 CQA Safety—sterility Safety—sterility Safety—sterility